DFT and Molecular Docking Studies of Melatonin and Some Analogues Interaction with Xanthine Oxidase as a Possible Antiradical Mechanism
DOI:
https://doi.org/10.29356/jmcs.v68i1.2072Keywords:
antiradical properties, Density Functional Theory, melatonin, xanthine oxidase, molecular dockingAbstract
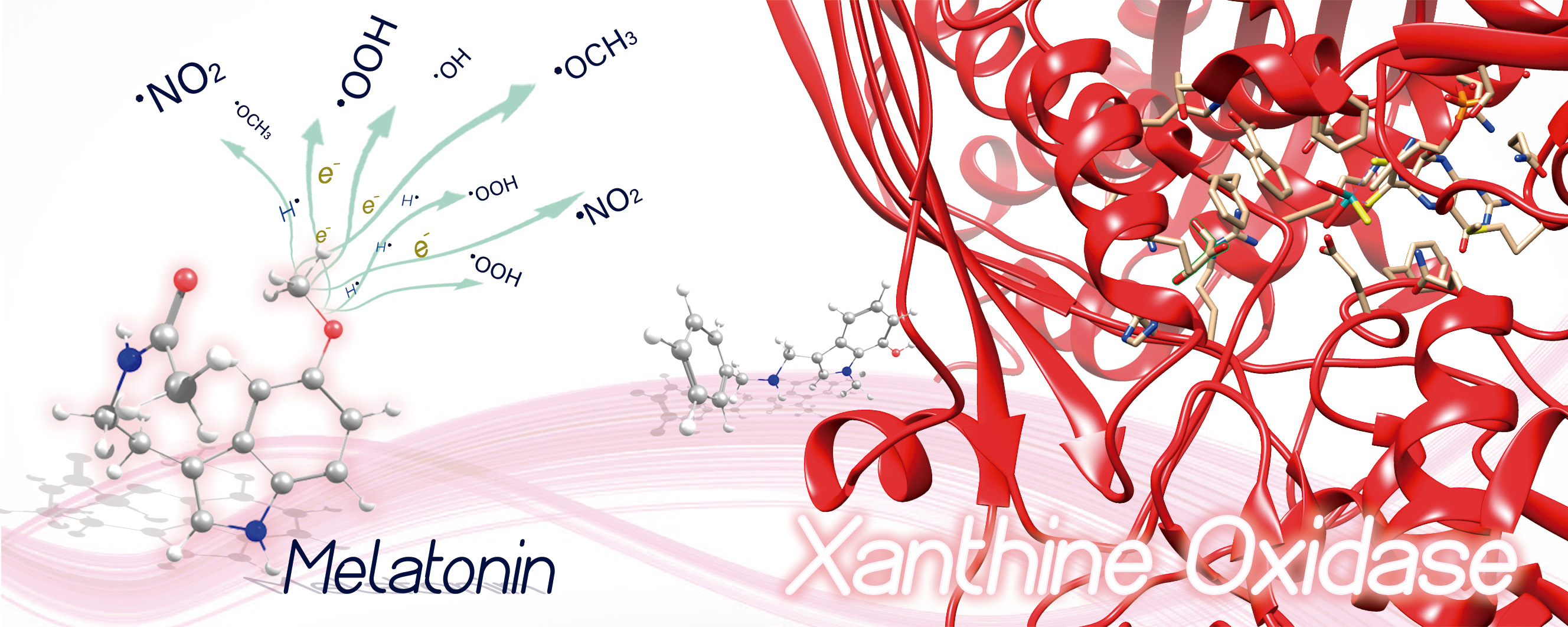
Melatonin (Mel) and some of its active metabolites such as N1-acetyl-5-methoxykynuramine (AMK), N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), 6-hydroxymelatonin (6OHM), and the analogues Ir and It recently designed by Galano's group, have been studied within density functional theory (DFT). The purpose is to evaluate some plausible mechanisms of action of melatonin's metabolites and analogues with the free radicals (FR): OH ̇, NO ̇2, HOO ̇, and CH3O͘ . We calculated global chemical reactivity descriptors from conceptual DFT to evaluate their antiradical properties. We used water and pentyl ethanoate as solvents to simulate the physiological conditions, modeled via the continuum solvation model based on density (SMD). We assess the following plausible mechanisms: single electrons transfer (SET), hydrogen atom transfer (HAT) and xanthine oxidase (XO) inhibition. We performed our calculations at the M06-2X/6-31+G* level of theory. The results indicate that Mel, AMK, AFMK, 6OHM, It, and Ir are good antiradicals towards the FRs: NO ̇2 and CH3O , while It and Ir could be suitable XO inhibitors.
Keywords: Antiradical properties; Density Functional Theory; melatonin; xanthine oxidase; molecular docking.
Resumen. La melatonina (Mel) y algunos de sus metabolitos activos como N1-acetil-5-metoxiquinuramina (AMK), N1-acetil-N2-formil-5-metoxiquinuramina (AFMK), 6-hidroximelatonina (6OHM) y los análogos Ir e It, diseñados recientemente por el grupo de Galano, han sido estudiados con la teoría de funcionales de la densidad (DFT). El propósito es evaluar algunos mecanismos de acción plausibles de los metabolitos y análogos de la melatonina con los radicales libres (FR):OH ̇, NO ̇2, HOO ̇ y CH3O ̇. Calculamos los descriptores de reactividad química global a partir de DFT conceptual para evaluar sus propiedades antirradicales. Usamos agua y etanoato de pentilo como solventes para simular las condiciones fisiológicas, modeladas a través del modelo continuo de solvatación basado en la densidad (SMD). Evaluamos los siguientes mecanismos plausibles: transferencia de electrones individuales (SET), transferencia de átomos de hidrógeno (HAT) e inhibición de la xantina oxidasa (XO). Realizamos nuestros cálculos al nivel de teoría M06-2X/6-31+G*. Los resultados indican que Mel, AMK, AFMK, 6OHM, It e Ir son buenos antirradicales frente a los FRs: NO ̇2 y CH3O ̇, mientras que It e Ir podrían ser inhibidores adecuados de XO.
Downloads
References
Dharmaraja, A. T. J. Med. Chem. 2017, 60, 3221–3240. DOI: https://doi.org/10.1021/acs.jmedchem.6b01243.
Kostić, D. A.; Dimitrijević, D. S.; Stojanović, G. S.; Palić, I. R.; Đorđević, A. S.; Ickovski, J. D. J. Chem. 2015, 2015, 294858. DOI: https://doi.org/10.1155/2015/294858.
Giorgi, C.; Marchi, S.; Simoes, I. C. M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Chapter Six. in: Mitochondria and Longevity; López-Otín, C., Galluzzi, L. B. T.-I. R. of C. and M. B., Eds.; Academic Press, 2018; 340, 209–344 DOI: https://doi.org/https://doi.org/10.1016/bs.ircmb.2018.05.006.
Phaniendra, A.; Jestadi, D. B.; Periyasamy, L. Indian J. Clin. Biochem. 2015, 30, 11–26. DOI: https://doi.org/10.1007/s12291-014-0446-0.
Alkadi, H. Infect. Disord. Drug. Targets. 2020, 20, 16–26. DOI: https://doi.org/10.2174/1871526518666180628124323.
Oroian, M.; Escriche, I. Int. Food Res. J. 2015, 74, 10–36. DOI: https://doi.org/10.1016/j.foodres.2015.04.018.
Jamshidi-kia, F.; Wibowo, J. P.; Elachouri, M.; Masumi, R.; Salehifard-Jouneghani, A.; Abolhasanzadeh, Z.; Lorigooini, Z. J. Herbmed. Pharmacol. 2020, 9, 191–199. DOI: https://doi.org/10.34172/jhp.2020.25.
Galano, A.; Tan, D. X.; Reiter, R. J. J. Pineal Res. 2011, 51, 1–16. DOI: https://doi.org/10.1111/j.1600-079X.2011.00916.x.
Galano, A. RSC Adv. 2016, 6, 22951–22963. DOI: https://doi.org/10.1039/c6ra00549g.
Poeggeler, B.; Saarela, S.; Reiter, R. J.; Tan, D. X.; Chen, L. D.; Manchester, L. C.; Barlow-Walden, L. R. Ann. N. Y. Acad. Sci. 1994, 738, 419–420. DOI: https://doi.org/10.1111/j.1749-6632.1994.tb21831.x.
Galano, A.; Reiter, R. J. J. Pineal Res. 2018, 65, e12514. DOI: https://doi.org/https://doi.org/10.1111/jpi.12514.
Álvarez-Diduk, R.; Galano, A.; Tan, D. X.; Reiter, R. J. Theor. Chem. Acc. 2016, 135, 38. DOI: https://doi.org/10.1007/s00214-015-1785-5.
Ramis, M. R.; Esteban, S.; Miralles, A.; Tan, D.-X.; Reiter, R. J. Curr. Med. Chem, 2015, 22, 2690–2711. DOI: https://doi.org/10.2174/0929867322666150619104143.
Galano, A.; Tan, D. X.; Reiter, R. J. J. Pineal Res. 2013, 54, 245–257. DOI: https://doi.org/10.1111/jpi.12010.
Álvarez-Diduk, R.; Galano, A.; Tan, D. X.; Reiter, R. J. J. Phys. Chem. B. 2015, 119, 8535–8543. DOI: https://doi.org/10.1021/acs.jpcb.5b04920.
Hardeland, R. Endocrine. 2005, 27, 119–130. DOI: https://doi.org/10.1385/endo:27:2:119.
Costa, J. D.; Ramos, R. D.; Costa, K. D.; Brasil, D. D.; Silva, C. H.; Ferreira, E. F.; Borges, R. D.; Campos, J. M.; Macêdo, W. J.; Santos, C. B. Mol. 2018. DOI: https://doi.org/10.3390/molecules23112801.
Guerra-Vargas, M. A.; Rosales-Hernández, M. C.; Martínez-Fonseca, N.; Padilla-Martínez, I.; Fonseca-Sabater, Y.; Martínez-Ramos, F. Med. Chem. Res. 2018, 27, 1186–1197. DOI: https://doi.org/10.1007/s00044-018-2139-3.
Kaçmaz, A.; User, E. Y.; Şehirli, A. Ö.; Tilki, M.; Ozkan, S.; Şener, G. Surg. Today. 2005, 35, 744–750. DOI: https://doi.org/10.1007/s00595-005-3027-2.
Okutan, H.; Savas, C.; Delibas, N. Interact. Cardiovasc. Thorac. Surg. 2004, 3, 519–522. DOI: https://doi.org/10.1016/j.icvts.2004.05.005.
Juan, C. A.; Pérez de la Lastra, J. M.; Plou, F. J.; Pérez-Lebeña, E. Int. J. Mol. Sci. 2021. DOI: https://doi.org/10.3390/ijms22094642.
Teixeira, A.; Morfim, M. P.; de Cordova, C. A. S.; Charão, C. C. T.; de Lima, V. R.; Creczynski-Pasa, T. B. J. Pineal Res. 2003, 35, 262–268. DOI: https://doi.org/https://doi.org/10.1034/j.1600-079X.2003.00085.x.
Zhou, J.; Zhang, S.; Zhao, X.; Wei, T. J. Pineal Res. 2008, 45, 157–165. DOI: https://doi.org/https://doi.org/10.1111/j.1600-079X.2008.00570.x.
Castañeda-Arriaga, R.; Pérez-González, A.; Reina, M.; Galano, A. Theor. Chem. Acc. 2020, 139, 1–12. DOI: https://doi.org/10.1007/s00214-020-02641-9.
Reina, M.; Castañeda-Arriaga, R.; Perez-Gonzalez, A.; Guzman-Lopez, E. G.; Tan, D.-X.; Reiter, R. J.; Galano, A. Melatonin Res. 2018, 1, 27–58. DOI: https://doi.org/10.32794/mr11250003.
Galano, A.; Raúl Alvarez-Idaboy, J. Int. J. Quantum. Chem. 2019, 119, e25665. DOI: https://doi.org/10.1002/qua.25665.
Reina, M.; Martínez, A. Comput. Theor. Chem. 2018, 1123, 111–118. DOI: https://doi.org/https://doi.org/10.1016/j.comptc.2017.11.017.
Galano, A. Phys. Chem. Chem. Phys. 2011, 13, 7178–7188. DOI: https://doi.org/10.1039/c0cp02801k.
Geerlings, P.; De Proft, F.; Langenaeker, W. Chem. Rev. 2003, 103, 1793–1874. DOI: https://doi.org/10.1021/cr990029p.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; et al. Gaussian 09. Revision C.01. Gaussian 09. Revision C.01, Gaussian, Inc, Wallingford CT. Gaussian, Inc.: Wallingford CT 2010.
Manzanilla, B.; Robles, J. J. Mol. Model. 2022, 28, 68. DOI: https://doi.org/10.1007/s00894-022-05056-4.
Cannington, P. H.; Ham, N. S. J. Electron. Spectrosc. Relat. Phenom. 1983, 32, 139–151. DOI: https://doi.org/10.1016/0368-2048(83)85092-0.
Marenich, A. V; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B. 2009, 113, 6378–6396. DOI: https://doi.org/10.1021/jp810292n.
Spartan, W. I. Spartan 08. Irvine, CA. 2008.
Halgren, T. A. J. Comput. Chem. 1996, 17, 490–519. DOI: https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P.
Halgren, T. A. J. Comput. Chem. 1996, 17, 520–552. DOI: https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<520::AID-JCC2>3.0.CO;2-W. DOI: https://doi.org/10.1002/(SICI)1096-987X(199604)17:6<520::AID-JCC2>3.3.CO;2-W
Halgren, T. A. J. Comput. Chem. 1996, 17, 553–586. DOI: https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<553::AID-JCC3>3.0.CO;2-T.
Halgren, T. A.; Nachbar, R. B. J. Comput. Chem. 1996, 17, 587–615. DOI: https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<587::AID-JCC4>3.0.CO;2-Q.
Ho, J.; Coote, M. L. Theor. Chem. Acc. 2009, 125, 3–21. DOI: https://doi.org/10.1007/s00214-009-0667-0.
Janak, J. F. Phys. Rev. B. 1978, 18, 7165–7168. DOI: https://doi.org/10.1103/PhysRevB.18.7165.
Casida, M. E. Phys. Rev. B. 1999, 59, 4694–4698. DOI: https://doi.org/10.1103/PhysRevB.59.4694.
Saha, B.; Bhattacharyya, P. K. RSC Adv. 2016, 6, 79768–79780. DOI: https://doi.org/10.1039/C6RA15016K.
Parr, R. G.; Pearson, R. G. J. Am. Chem. Soc. 1983, 105, 7512–7516. DOI: https://doi.org/10.1021/ja00364a005.
Yang, W.; Parr, R. G. PNAS. 1985, 82, 6723–6726 DOI: https://doi.org/10.1073/pnas.82.20.6723.
Parr, R. G.; Yang, W. J. Am. Chem. Soc. 1984, 106, 4049–4050. DOI: https://doi.org/10.1021/ja00326a036.
Gázquez, J. L.; Cedillo, A.; Vela, A. J. Phys. Chem. A. 2007, 111, 1966–1970. DOI: https://doi.org/10.1021/jp065459f.
Duarte Ramos Matos, G.; Kyu, D. Y.; Loeffler, H. H.; Chodera, J. D.; Shirts, M. R.; Mobley, D. L. J. Chem. Eng. Data. 2017, 62,1559–1569. DOI: https://doi.org/10.1021/acs.jced.7b00104.
Leo, A. J. Chem. Rev. 1993, 93, 1281–1306. DOI: https://doi.org/10.1021/cr00020a001.
Spartan, W. I. Spartan 18. Irvine, CA. 2018.
Ghose, A. K.; Pritchett, A.; Crippen, G. M. J. Comput. Chem. 1988, 9, 80–90. DOI: https://doi.org/10.1002/jcc.540090111.
Martínez, A.; Vargas, R.; Galano, A. J. Phys. Chem. B. 2009, 113, 12113–12120. DOI: https://doi.org/10.1021/jp903958h.
Enroth, C.; Eger, B. T.; Okamoto, K.; Nishino, T.; Nishino, T.; Pai, E. F. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 10723–10728. DOI: https://doi.org/10.1073/pnas.97.20.10723.
Morris, G. M.; Huey, R.; Lindstrom, W.; Sanner, M. F.; Belew, R. K.; Goodsell, D. S.; Olson, A. J. J. Comput. Chem. 2009, 30, 2785–2791. DOI: https://doi.org/10.1002/jcc.21256.
Morris, G. M.; Goodsell, D. S.; Halliday, R. S.; Huey, R.; Hart, W. E.; Belew, R. K.; Olson, A. J. J. Comput. Chem. 1998, 19, 1639–1662. DOI: https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B.
BIOVIA. Discovery Studio Visualizer. Dassault Systèmes: San Diego 2020.
Mahal, H. S.; Sharma, H. S.; Mukherjee, T. Free Radic. Biol. Med. 1999, 26, 557–565. DOI: https://doi.org/10.1016/s0891-5849(98)00226-3.
Galano, A. A. Theor. Chem. Acc. 2016, 135, 157. DOI: https://doi.org/10.1007/s00214-016-1917-6.
Domingo, L. R.; Aurell, M. J.; Pérez, P.; Contreras, R. Tetrahedron. 2002, 58, 4417–4423. DOI: https://doi.org/https://doi.org/10.1016/S0040-4020(02)00410-6.
Johns, J. R.; Platts, J. A. Org. Biomol. Chem. 2014, 12, 7820–7827. DOI: https://doi.org/10.1039/c4ob01396d.
Galano, A.; Tan, D. X.; Reiter, R. J. RSC Adv. 2014, 4, 5220–5227. DOI: https://doi.org/10.1039/c3ra44604b.
Romero, Y.; Martínez, A. J. Mol. Model. 2015, 21, 220. DOI: https://doi.org/10.1007/s00894-015-2773-3.
Chung, H. Y.; Baek, B. S.; Song, S. H.; Kim, M. S.; Huh, J. I.; Shim, K. H.; Kim, K. W.; Lee, K. H. Age (Omaha). 1997, 20, 127–140. DOI: https://doi.org/10.1007/s11357-997-0012-2.
Lin, H.-C.; Tsai, S.-H.; Chen, C.-S.; Chang, Y.-C.; Lee, C.-M.; Lai, Z.-Y.; Lin, C.-M. Biochem. Pharmacol. 2008, 75, 1416–1425. DOI: https://doi.org/10.1016/j.bcp.2007.11.023.
Kontoyianni, M.; McClellan, L. M.; Sokol, G. S. J. Med. Chem. 2004, 47, 558–565. DOI: https://doi.org/10.1021/jm0302997.
Chen, Y.; Gao, Y.; Wu, F.; Luo, X.; Ju, X.; Liu, G. New J. Chem. 2020, 44, 19276–19287. DOI: https://doi.org/10.1039/D0NJ03221B.
Shen, L.; Ji, H.-F. Bioorg. Med. Chem. Lett. 2009, 19, 5990–5993. DOI: https://doi.org/10.1016/j.bmcl.2009.09.076.
Fatima, I.; Zafar, H.; Khan, K. M.; Saad, S. M.; Javaid, S.; Perveen, S.; Choudhary, M. I. Bioorg. Chem. 2018, 79, 201–211. DOI: https://doi.org/10.1016/j.bioorg.2018.04.021.
Santi, M. D.; Paulino Zunini, M.; Vera, B.; Bouzidi, C.; Dumontet, V.; Abin-Carriquiry, A.; Grougnet, R.; Ortega, M. G. Eur. J. Org. Chem. 2018, 143, 577–582. DOI: https://doi.org/10.1016/j.ejmech.2017.11.071.

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2023 Brenda Manzanilla, Minerva Martinez-Alfaro, Juvencio Robles

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








