Regioselective Functionalization and Diels–Alder Cycloadditions of Exocyclic Dienes in Five-membered Heterocycles
DOI:
https://doi.org/10.29356/jmcs.v69i4.2343Keywords:
4,5-dimethylene-2-oxazolidinone dienes, 5-functionalized 4-oxazolin-2-ones, 4,5-dimethylene-2-imidazolidinone dienes, Staunton-Weinreb annulation, Diels–Alder reactionAbstract
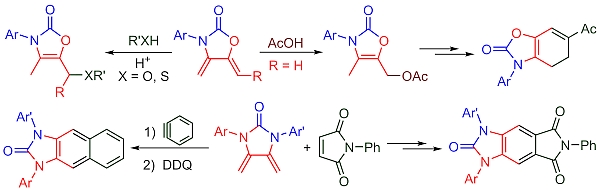
Abstract. An acid-catalyzed regioselective addition of diverse nucleophiles to exo-oxazolidin-2-one dienes was presently carried out, leading to a series of functionalized 4-oxazolin-2-ones. The direct formylation of 4-methyl-4-oxazolin-2-ones provided the corresponding 5-formyl-4-oxazolin-2-ones, which were applied in the construction of the 4,5-dihydrobenzo[d]oxazolones through a Staunton-Weinreb annulation process. The reactivity of symmetrical and unsymmetrical exo-imidazolidin-2-one dienes was studied in Diels–Alder cycloadditions with dienophiles N-phenylmaleimide and benzyne. The aromatization of the [4+2] adducts led to the polycyclic benzo- and naphtho[d]imidazol-2-ones, which have potential pharmacological activity.
Resumen. Se describe la adición regioselectiva catalizada por ácido de diversos nucleófilos a los dienos exo-oxazolidinonas que conduce a una serie de 4-oxazolin-2-onas funcionalizadas. La formilación directa de 4-metil-4-oxazolidin-2-onas proporcionó las 5-formil-4-oxazolin-2-onas correspondientes, las cuales se emplearon en la construcción de las 4,5-dihidrobenzo[d]oxazolonas mediante un proceso de anillación de Staunton-Weinreb. Se estudió la reactividad de dienos exo-imidazolidin-2-onas simétricos y no simétricos en cicloadiciones de Diels-Alder con los dienófilos N-fenilmaleimida y bencino. La aromatización de los aductos [4+2] condujo a los benzo- y nafto[d]imidazol-2-onas policíclicas como compuestos con actividad farmacológica potencial.
Downloads
References
1. (a) Sauer, J.; Sustmann, R. Angew. Chem., Int. Ed. Engl. 1980, 19, 779–807. DOI: https://doi.org/10.1002/anie.198997791; (b) Pindur, U.; Lutz, G.; Otto, C. Chem. Rev. 1993, 93, 741–761. DOI: https://doi.org/10.1021/cr00018a006; (c) Herges, R.; Jiao, H.; von Ragué Schleyer, P. Angew. Chem., Int. Ed. Engl. 1994, 33, 1376–1378. DOI: https://doi.org/10.1002/anie.199413761; (d) Diedrich, M. K.; Klärner, F.-G. J. Am. Chem. Soc. 1998, 120, 6212–6218. DOI: https://doi.org/10.1021/ja973936p; (e) Suárez, D.; Sordo, J. A. Chem. Commun. 1998, 385–386. DOI: https://doi.org/10.1039/A707086A; (f) Brocksom, T. J.; Nakamura, J.; Ferreira, M. L.; Brocksom, U. J. Braz. Chem. Soc. 2001, 12, 597–622. DOI: https://doi.org/10.1590/S0103-50532001000500004; (g) Kumar, A. Chem. Rev. 2001, 101, 1–19. DOI: https://doi.org/10.1021/cr990410; (h) Tantillo, D. J.; Houk, K. N.; Jung, M. E. J. Org. Chem. 2001, 66, 1938–1940. DOI: https://doi.org/10.1021/jo001172h; (i) Ayers, P. W.; Morell, C.; De Proft, F.; Geerlings, P. Chem. Eur. J. 2007, 13, 8240–8247. DOI: https://doi.org/10.1002/chem.200700365; (j) Domingo, L. R.; Sáez, J. A. Org. Biomol. Chem. 2009, 7, 3576–3583. DOI: https://doi.org/10.1039/B909611F; (k) Wang, Z.; Hirschi, J. S.; Singleton, D. A. Angew. Chem. Int. Ed. 2009, 48, 9156–9159. DOI: https://doi.org/10.1002/anie.200903293; (l) Ishihara, K.; Sakakura, A. Intermolecular Diels-Alder Reactions, in Comprehensive Organic Synthesis; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, 2014; Vol. 5, Chap. 5.09, 351–408.
2. (a) Patman, R. L.; Bower, J. F.; Kim, I. S.; Krische, M. J. Aldrichim. Acta 2008, 41, 95–104; (b) Longo, P.; Pragliola, S.; Milano, G.; Guerra, G. J. Am. Chem. Soc. 2003, 125, 4799–4803. DOI: https://doi.org/10.1021/ja028462; (c) Li, Y.; Chen, J.; Ng, J. J. W.; Chiba, S. Angew. Chem. Int. Ed.. 2023, 62, e202217735. DOI: https://doi.org/10.1002/anie.202217735.
3. (a) Gleiter, R.; Böhm, M. C. Pure Appl. Chem. 1983, 55, 237–244. DOI: https://doi.org/10.1351/pac198855020237; (b) Kahn, S. D.; Pau, C. F.; Overman, L. E.; Hehre, W. J. J. Am. Chem. Soc. 1986, 108, 7381–7396. DOI: https://doi.org/10.1021/ja00283a038; (c) Houk, K. N.; Li, Y.; Evanseck, J. D. Angew. Chem., Int. Ed. Engl. 1992, 31, 682–708. DOI: https://doi.org/10.1002/anie.199206821; (d) Damoun, S.; Van de Woude, G.; Méndez, F.; Geerlings, P. J. Phys. Chem. A 1997, 101, 886–893. DOI: https://doi.org/10.1021/jp9611840; (e) Xidos, J. D.; Poirier, R. A.; Pye, C. C.; Burnell, D. J. J. Org. Chem. 1998, 63, 105–112. DOI: https://doi.org/10.1021/jo9712815; (f) García, J. I.; Martínez-Merino, V.; Mayoral, J. A.; Salvatella, L. J. Am. Chem. Soc. 1998, 120, 2415–2420. DOI: https://doi.org/10.1021/ja97282279; (g) Kong, S.; Evanseck, J. D. J. Am. Chem. Soc. 2000, 122, 10418–10427. DOI: https://10.1021/ja0010249; (h) Quadrelli, P.; Romano, S.; Toma, L.; Caramella, P. J. Org. Chem. 2003, 68, 6035–6038. DOI: https://10.1021/jo034401j; (i) Çelebi-Ölçüm, N.; Ess, D. H.; Aviyente, V.; Houk, K. N. J. Org. Chem. 2008, 73, 7472–7480. DOI: https://10.1021/jo801076t; (j) Domingo, L. R.; Chamorro, E.; Pérez, P. Org. Biomol. Chem. 2010, 8, 5495–5504. DOI: https://10.1039/c0ob00563k; (k) Ramírez-Gualito, K.; López-Mora, N.; Jiménez-Vázquez, H. A.; Tamariz, J.; Cuevas, G. J. Mex. Chem. Soc. 2013, 57, 267–275. DOI: https://10.29356/jmcs.v57i4.189; (l) Jasiński, R.; Kubik, M.; Łapczuk-Krygier, A.; Kącka, A.; Dresler, E.; Boguszewska-Czubara, A. Reac. Kinet. Mech. Cat. 2014, 113, 333–345. DOI: https://10.1007/s11144-014-0753-8; (m) Mlostoń, G.; Urbaniak, K.; Sobiecka, M.; Heimgartner, H.; Würthwein, E.-U.; Zimmer, R.; Lentz, D.; Reissig, H.-U. Molecules 2021, 26, 2544. DOI: https://10.3390/molecules26092544.
4. (a) Carruthers, W., in: Cycloaddition Reactions in Organic Synthesis; Pergamon Press: Oxford, 1990; (b) Oppolzer, W., in: Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I.; Paquette, L. A., Eds.; Pergamon Press: Oxford, 1991; Vol. 5, Chapter 4.1; (c) Fringuelli, F.; Taticchi, A. The Diels-Alder Reaction: Selected Practical Methods; J. Wiley & Sons: Chichester, 2002; (d) Corey, E. J. Angew. Chem. Int. Ed. 2002, 41, 1650–1667. DOI: https://doi.org/10.1002/1521-3773(20020517)41:10<1650::AID-ANIE1650>3.0.CO;2-B; (e) Nicolaou, K. C.; Snyder, S. A.; Montagnon, T.; Vassilikogiannakis, G. Angew. Chem. Int. Ed. 2002, 41, 1668–1698, and references cited therein. DOI: https://doi.org/10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z; (f) Reymond, S.; Cossy, J. Chem Rev. 2008, 108, 5359–5406. DOI: https://doi.org/10.1021/cr078346g; (g) Wang, J.; Ma, D. Angew. Chem. Int. Ed. 2019, 58, 15731–15735. DOI: https://doi.org/10.1002/anie.201909349; (h) Schwinger, D. P.; Peschel, M. T.; Jaschke, C.; Jandl, C.; de Vivie-Riedle, R.; Bach, T. J. Org. Chem. 2022, 87, 4838–4851. DOI: https://doi.org/10.1021/acs.joc.2c00186; (i) Ghosh, S.; Erchinger, J. E.; Maji, R.; List, B. J. Am. Chem. Soc. 2022, 144, 6703–6708. DOI: http://doi.org/10.1021/jacs.2c01971; (j) Li, L.-X.; Min, L.; Yao, T.-B.; Ji, S.-X.; Qiao, C.; Tian, P.-L.; Sun, J.; Li, C.-C. J. Am. Chem. Soc. 2022, 144, 18823–18828. DOI: https://doi.org/10.1021/jacs.2c09548.
5. (a) Wiersum, U. E. Aldrichimica Acta 1981, 14, 53–58; (b) Fringuelli, F.; Taticchi, A., in: Dienes in the Diels-Alder Reaction; J. Wiley & Sons: New York, 1990; (c) Martin, N.; Seoane, C.; Hanack, M. Org. Prep. Proc. Int. 1991, 23, 237–272. DOI: https://doi.org/10.1080/00304949109458320; (d) Manoharan, M.; De Proft, F.; Geerlings, P. J. Org. Chem. 2000, 65, 7971–7976. DOI: https://doi.org/10.1021/jo001156k; (e) Alcaide, B.; Almendros, P.; Aragoncillo, C. Chem. Soc. Rev. 2014, 43, 3106–3135 DOI: https://doi.org/10.1039/c3cs60462d.
6. (a) Haber, M.; Pindur, U. Tetrahedron 1991, 47, 1925–1936. DOI: https://doi.org/S0040-4020(01)96104-6; (b) Ruiz, N.; Pujol, M. D.; Guillaumet, G.; Coudert, G. Tetrahedron Lett. 1992, 33, 2965–2968. DOI: https://doi.org/S0040-4039(00)79573-6; (c) Chaloner, L. M.; Crew, A. P. A.; O’Neill, P. M.; Storr, R. C.; Yelland, M. Tetrahedron 1992, 37, 8101–8116. DOI: https://doi.org/S0040-4020(01)80480-4; (d) Chou, T. S.; Chang, R. C. J. Org. Chem. 1993, 58, 493–496. DOI: https://doi.org/jo00054a037; (e) Hercouet, A.; Berrée, F.; Lin, C. H.; Toupet, L.; Carboni, B. Org. Lett. 2007, 9, 1717–1720. DOI: https://doi.org/10.1021/ol070400s; (f) Samanta, S.; Mohapatra, H.; Jana, R.; Ray, J. K. Tetrahedron Lett. 2008, 49, 7153–7156. DOI: https://doi.org/10.1016/j.tetlet.2008.09.162; (g) Inagaki, F.; Mizutani, M.; Kuroda, N.; Mukai, C. J. Org. Chem. 2009, 74, 6402–6405. DOI: https://doi.org/10.1021/jo901325d; (h) Zhou, L.; Zhang, M.; Li, W.; Zhang, J. Angew. Chem. Int. Ed. 2014, 53, 6542–6545. DOI: https://doi.org/10.1002/anie.201403709; (i) Li, G.-N.; Chen, G.-Y.; Niu, Z.-G.; Lei, B.-X.; Feng, H.-J. J. Heterocycl. Chem. 2014, 51, E367–E371. DOI: https://doi.org/10.1002/jhet.1945; (j) Hirata, G.; Yamada, N.; Sanada, S.; Onodera, G.; Kimura, M. Org. Lett. 2015, 17, 600–603. DOI: https://doi.org/10.1021/ol503614d.
7. (a) Mandal, A. B.; Gómez, A.; Trujillo, G.; Méndez, F.; Jiménez, H. A.; Rosales, M. J.; Martínez, R.; Delgado, F.; Tamariz, J. J. Org. Chem. 1997, 62, 4105–4115. DOI: https://doi.org/10.1021/jo962403g; (b) Fuentes, A.; Martínez-Palou, R.; Jiménez-Vázquez, H. A.; Delgado, F.; Reyes, A.; Tamariz, J. Monatsh. Chem. 2005, 136, 177–192. DOI: https://doi.org/10.1007/s00706-004-0244-0.
8. Martínez, R.; Jiménez-Vázquez, H. A.; Reyes, A.; Tamariz, J. Helv. Chim. Acta 2002, 85, 464–482. DOI: https://doi.org/10.1002/1522-2675(200202)85:2<464::AID-HLCA464>3.0.CO;2-U.
9. Martínez, R.; Jiménez-Vázquez, H. A.; Delgado, F.; Tamariz, J. Tetrahedron 2003, 59, 481–492. DOI: https://doi.org/10.1016/S0040-4020(02)01536-3.
10. González-Romero, C.; Bernal, P.; Jiménez, F.; Cruz, M. C.; Fuentes-Benites, A.; Benavides, A.; Bautista, R.; Tamariz, J. Pure Appl. Chem. 2007, 79, 181–191. DOI: https://doi.org/10.1351/pac200779020181.
11. Mandal, A. B.; Delgado, F.; Tamariz, J. Synlett 1998, 87–89. DOI: https://doi.org/10.1055/s-1998-1571.
12. (a) Bautista, R.; Benavides, A.; Jiménez-Vázquez, H. A.; Tamariz, J. Nat. Prod. Res. 2013, 27, 1749–1756, and references cited therein. DOI: https://doi.org/10.1080/14786419.2012.751599; (b) Ávila-Melo, J. L.; Benavides, A.; Fuentes-Gutiérrez, A.; Tamariz, J.; Jiménez-Vázquez, H. A. Synthesis 2021, 53, 2201–2211. DOI: https://doi.org/10.1055/a-1385-9052.
13. Ortega-Jiménez, F.; Benavides, A.; Delgado, F.; Jiménez-Vázquez, H. A.; Tamariz, J. Organometallics 2010, 29, 149–159. DOI: https://doi.org/10.1021/om900772z.
14. Reyes, L.; Mendoza, H.; Vázquez, M. A.; Ortega-Jiménez, F.; Fuentes-Benítes, A.; Flores-Conde, M. I.; Jiménez-Vázquez, H.; Miranda, R.; Tamariz, J.; Delgado, F. Organometallics 2008, 27, 4334–4345. DOI: https://doi.org/10.1021/om8002416.
15. Santoyo, B. M.; González‐Romero, C.; Merino, O.; Martínez‐Palou, R.; Fuentes‐Benites, A.; Jiménez‐Vázquez, H. A.; Delgado, F.; Tamariz, J. Eur. J. Org. Chem. 2009, 2505–2518. DOI: https://doi.org/10.1002/ejoc.200900114.
16. Merino, O.; Santoyo, B. M.; Montiel, L. E.; Jiménez-Vázquez, H. A.; Zepeda, L. G.; Tamariz, J. Tetrahedron Lett. 2010, 51, 3738–3742. DOI: https://doi.org/10.1016/j.tetlet.2010.05.034.
17. Zárate-Zárate, D.; Aguilar, R.; Hernández-Benitez, R. I.; Labarrios, E. M.; Delgado, F.; Tamariz, J. Tetrahedron. 2015, 71, 6961–6978. DOI: https://doi.org/10.1016/j.tet.2015.07.010.
18. Santoyo, B. M.; González‐Romero, C.; Zárate‐Zárate, D.; Hernández‐Benitez, R. I.; Pelayo, V.; Barrera, E.; Escalante, C. H.; Fuentes‐Benites, A.; Martínez‐Morales, G.; López, J.; Vázquez, M. A.; Delgado, F.; Jiménez‐Vázquez, H. A.; Tamariz, J. Chirality 2019, 31, 719–749. DOI: https://doi.org/10.1002/chir.23109.
19. Barrera, E.; Hernández-Benitez, R. I.; González-González, C. A.; Escalante, C. H.; Fuentes-Benites, A.; González-Romero, C.; Becerra-Martínez, E.; Delgado, F.; Tamariz, J. Eur. J. Org. Chem. 2022, 2022, e202200364. DOI: https://doi.org/10.1002/ejoc.202200364.
20. Yescas-Galicia, D.; Restrepo-Osorio, R. A.; García-González, A. N.; Hernández-Benítez, R. I.; Espinoza-Hicks, J. C.; Escalante, C. H.; Barrera, E.; Santoyo, B. M.; Delgado, F.; Tamariz, J. J. Org. Chem. 2022, 87, 13034–13052. DOI: https://doi.org/10.1021/acs.joc.2c01563.
21. Bautista, R.; Bernal, P.; Herrera, R.; Santoyo, B. M.; Lazcano-Seres, J. M.; Delgado, F.; Tamariz, J. J. Org. Chem. 2011, 79, 7901–7911. DOI: https://doi.org/10.1021/jo201335y.
22. Espinoza-Hicks, C.; Montoya, P.; Bautista, R.; Jiménez-Vázquez, H. A.; Rodríguez-Valdez, L. M.; Camacho-Dávila, A. A.; Cossío, F. P.; Delgado, F.; Tamariz, J. J. Org. Chem. 2018, 83, 5347–5364. DOI: https://doi.org/10.1021/acs.joc.7b02344.
23. Rémond, G.; Portevin, B.; Bonnet, J.; Canet, E.; Regoli, D.; De Nanteuil, G. Eur. J. Med. Chem. 1997, 32, 843–868. DOI: https://doi.org/10.1016/S0223-5234(97)82771-7.
24. Edvinsson, L.; Sams, A.; Jansen-Olesen, I.; Tajti, J.; Kane, S. A.; Rutledge, R. Z.; Koblan, K. S.; Hill, R. G.; Longmore, J. Eur. J. Pharmacol. 2001, 415, 39–44. DOI: https://doi.org/10.1016/S0014-2999(00)00934-1.
25. Tapia, I.; Alonso-Cires, L.; López-Tudanca, P. L.; Mosquera, R.; Labeaga, L.; Innerárity, A.; Orjales, A. J. Med. Chem. 1999, 42, 2870–2880. DOI: https://doi.org/10.1021/jm981098j.
26. (a) Zhang, P.; Terefenko, E. A.; Wrobel, J.; Zhang, Z.; Zhu, Y.; Cohen, J.; Marschke, K. B.; Mais, D. Bioorg. Med. Chem. Lett. 2001, 11, 2747–2750. DOI: https://doi.org/10.1016/S0960-894X(01)00554-6; (b) Terefenko, E. A.; Kern, J.; Fensome, A.; Wrobel, J.; Zhu, Y.; Cohen, J.; Winneker, R.; Zhang, Z.; Zhang, P. Bioorg. Med. Chem. Lett. 2005, 15, 3600–3603. DOI: https://doi.org/10.1016/j.bmcl.2005.05.082.
27. Li, Q.; Li, T.; Woods, K. W.; Gu. W.-Z.; Cohen, J.; Stoll, V. S.; Galicia, T.; Hutchins, C.; Frost, D.; Rosenberg, S. H.; Sham, H. L. Bioorg. Med. Chem. Lett. 2005, 15, 2918–2922. DOI: https://doi.org/10.1016/j.bmcl.2005.03.049.
28. Martínez, R.; Jiménez-Vázquez, H. A.; Tamariz, J. Tetrahedron. 2000, 56, 3857–3866. DOI: https://doi.org/10.1016/S0040-4020(00)00311-2.
29. Fleming, I., in: Molecular orbitals and organic chemical reactions. Reference Edition. John Wiley & Sons: Chichester, UK, 2010.
30. Carrol, F. A., in: Perspectives on structure and mechanism in organic chemistry. John Wiley & Sons: New Jersey, 2010.
31. Toda, Y.; Gomyou, S.; Tanaka, S.; Komiyama, Y.; S.; Kikuchi, A.; Suga, H, Org. Lett. 2017, 19, 5786–5789. DOI: https://doi.org/10.1021/acs.orglett.7b02722; (b) Toda, Y.; Tanaka, S.; Gomyou, S.; Kikuchi, A.; Suga, H. Chem. Commun. 2019, 55, 5761–5764. DOI: https://doi.org/10.1039/C9CC01983A.
32. Tojo, G.; Fernández, M. Oxidation of alcohols to aldehydes and ketones. A guide to current common practice. Springer Science & Business Media: USA, 2006.
33. Donner, C. D. Tetrahedron. 2013, 69, 3747–3773. DOI: https:// doi.org/10.1016/j.tet.2013.03.034.
34. (a) Gilchrist, T. L. Supplement C: The chemistry of triple bonded functional groups, Part 1. Patai, S; Rappoport, Z. (Eds.). John Wiley and Sons: New York, 1983; (b) Wentrup, C. Aust. J. Chem. 2010, 63, 979–986. DOI: https:// doi.org/10.1071/CH10179.
35. Holden, C.; Greaney, M. F. Angew. Chem. Int. Ed. 2014, 53, 5746–5749. DOI: https://10.1002/anie.201402405.
36. (a) Gampe, C. M.; Carreira, E. M. Angew. Chem. Int. Ed. 2012, 51, 3766–3778. DOI: https://10.1002/anie.201107485; (b) Tadross, P. M.; Stoltz, B. M. Chem. Rev. 2012, 112, 3550–3577. DOI: https://10.1021/cr200478h.

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2025 Gustavo A. Monroy-Flores, Pablo Montoya, Ailyn N. García-González, Carlos H. Escalante, R. Uri Gutiérrez, R. Israel Hernández-Benitez, Aydeé Fuentes-Benítes, Edson Barrera, Omar Gómez-García, Francisco Delgado, Joaquín Tamariz

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









