DFT Calculation, ADME/T and Molecular Docking Approach of Methyl 2-oxo-1,2-dihydrofuro[3,4-d] pyrimidine-3(4H)carboxylate
DOI:
https://doi.org/10.29356/jmcs.v68i3.1995Keywords:
Dihydrofuro [3,4-d] pyrimidine, DFT/B3LYP, molecular docking, swiss ADME, ADMETAbstract
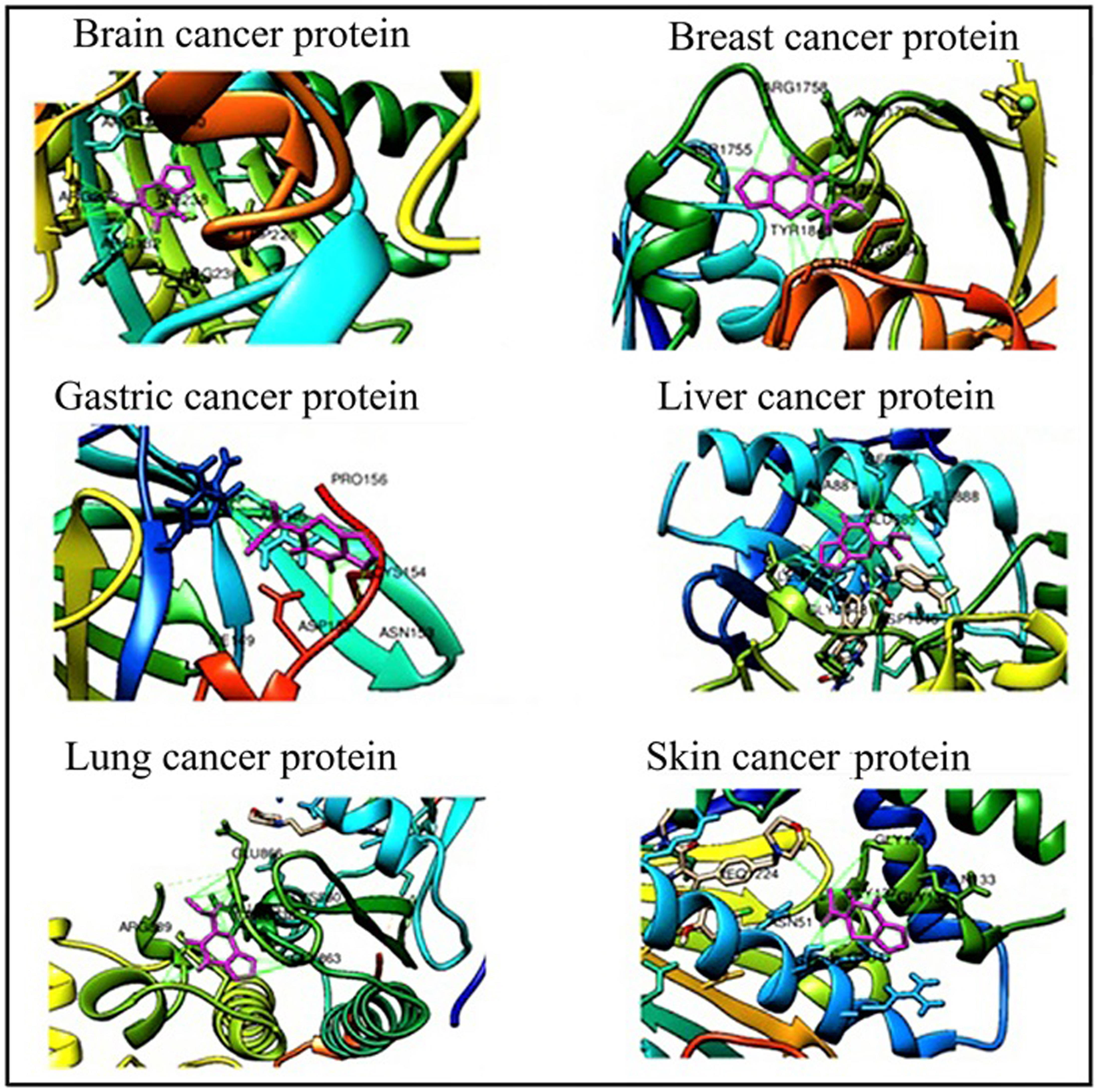
Abstract. The optimized geometry of methyl 2-oxo-1,2-dihydrofuro[3,4-d] pyrimidine-3(4H) carboxylate (FP) was determined by density functional theory calculations. Geometric properties of FP such as bond length, bond angle, dihedral bond angle, and HOMO-LUMO energies in the gas phase were calculated by using the Gaussian program. Delocalization of the molecule’s charge was analyzed using Mulliken Population Analysis (MPA) and Natural Population Analysis (NPA) approaches. Electrophilic and nucleophilic regions of FP were identified by drawing a molecular electrostatic potential map. NMR and FTIR spectra were calculated with the B3LYP and 6-311++G (2d, p) basis set and a detailed FTIR analysis was performed by using the VEDA program. To determine the consistency of the calculated NMR and FTIR spectra, they were compared with their corresponding experimental NMR and FTIR spectra. Molecular insertion studies of FP with six different cancer proteins were analyzed and their interactions were evaluated. Data on the pharmacokinetics and drug affinity of FP were obtained through the Swiss ADME and ADMET programs.
Resumen. Se optimizó la geometría del metil 2-oxo-1,2-dihidrofuro[3,4-d] pirimidina-3(4H) carboxilato (FP) por medio de la teoría de funcionales de la densidad. Utilizando el programa Gaussian, se calcularon en fase gas las propiedades geométricas del FP como longitudes de enlace, ángulos de enlace, ángulos diedros, y la diferencia de energías entre HOMO y LUMO. Se analizó la deslocalización de la carga en la molécula utilizando los análisis de población de Mulliken (MPA) y de población natural (NPA). Se identificaron las regiones electrofílicas y nucleofílicas mediante mapas del potencial electrostático molecular. Utilizando el funcional B3LYP y la base 6-311++G (2d, p) se calcularon los espectros de NMR y FTIR; se realizó un análisis detallado de los espectros de FTIR utilizando el programa VEDA. Para determinar la confiabilidad de los espectros calculados de NMR y FTIR, se compararon con los resultados experimentales. Se analizaron estudios de inserción molecular del FP a seis diferentes proteínas involucradas en cáncer para determinar sus interacciones. Utilizando los programas Swiss ADME y ADMET se determinaron la farmacocinética y la afinidad del FP.
Downloads
References
Abdel-Aziz, S. A.; Taher, E. S.; Lan, P.; Asaad, G. F.; Gomaa, H. A.; El-Koussi, N. A.; Youssif, B. G. Bioorg. Chem. 2021, 111, 104890. DOI: https://doi.org/10.1016/j.bioorg.2021.104890.
Aksinenko, A. Y.; Goreva, T. V.; Epishina, T. A.; Trepalin, S. V.; Sokolov, V. B. J. Fluor. Chem. 2016, 188, 191-195. DOI: https://doi.org/10.1016/j.jfluchem.2016.06.019.
Ballesteros-Casallas, A.; Paulino, M.; Vidossich, P.; Melo, C.; Jiménez, E.; Castillo, J.-C.; Portilla, J.; Miscione, G. P. Eur. J. Med. Chem. 2022, 4, 100028. DOI: https://doi.org/10.1016/j.ejmcr.2021.100028.
Basyouni, W. M.; Abbas, S. Y.; El‐Bayouki, K. A.; Dawood, R. M.; El Awady, M. K.; Abdelhafez, T. H. J. Heterocycl. Chem. 2021, 58, 1766-1774. DOI: https://doi.org/10.1002/jhet.4307.
Elkanzi, N. A. A. Orient. J. Chem. 2020, 36, 1001-1015. DOI: http://dx.doi.org/10.13005/ojc/360602. DOI: https://doi.org/10.13005/ojc/360602
Ali, F.; Khan, K. M.; Salar, U.; Iqbal, S.; Taha, M.; Ismail, N. H.; Perveen, S.; Wadood, A.; Ghufran, M.; Ali, B. Bioorg. Med. Chem. 2016, 24, 3624-3635. DOI: https://doi.org/10.1016/j.bmc.2016.06.002.
Katariya, K. D.; Reddy, D. V. J. Mol. Struct. 2022, 1253, 132240. DOI: https://doi.org/10.1016/j.molstruc.2021.132240.
Lamie, P. F.; Philoppes, J. N. Bioorg. Chem. 2021, 116, 105335. DOI: https://doi.org/10.1016/j.bioorg.2021.105335.
Manzoor, S.; Prajapati, S. K.; Majumdar, S.; Raza, M. K.; Gabr, M. T.; Kumar, S.; Pal, K.; Rashid, H.; Kumar, S.; Krishnamurthy, S. Eur. J. Med. Chem. 2021, 215, 113224. DOI: https://doi.org/10.1016/j.ejmech.2021.113224.
Raju, K. S.; AnkiReddy, S.; Sabitha, G.; Krishna, V. S.; Sriram, D.; Reddy, K. B.; Sagurthi, S. R. Bioorg. Med. Chem. Lett. 2019, 29, 284-290. DOI: https://doi.org/10.1016/j.bmcl.2018.11.036.
Fei, X.; Wang, J.-Q.; Miller, K. D.; Sledge, G. W.; Hutchins, G. D.; Zheng, Q.-H. Nucl. Med. Biol. 2004, 31, 1033-1041. DOI: https://doi.org/10.1016/j.nucmedbio.2004.02.006.
Scappini, B.; Gianfaldoni, G.; Caracciolo, F.; Mannelli, F.; Biagiotti, C.; Romani, C.; Pogliani, E. M.; Simonetti, F.; Borin, L.; Fanci, R. Am. J. Hematol. 2012, 87, 1047-1051. DOI: https://doi.org/10.1002/ajh.23308.
Halbrook, C. J.; Pontious, C.; Kovalenko, I.; Lapienyte, L.; Dreyer, S.; Lee, H.-J.; Thurston, G.; Zhang, Y.; Lazarus, J.; Sajjakulnukit, P. Cell Metab. 2019, 29, 1390-1399. e6. DOI: https://doi.org/10.1016/j.cmet.2019.02.001.
Verissimo, L. M.; Cabral, I.; Cabral, A. M.; Utzeri, G.; Veiga, F. J.; Valente, A. J.; Ribeiro, A. C. J. Chem. Thermodyn. 2021, 161, 106533. DOI: https://doi.org/10.1016/j.jct.2021.106533.
Abd El-Mageed, M. M.; Eissa, A. A.; Farag, A. E.-S.; Osman, E. E. A. Bioorg. Chem. 2021, 116, 105336. DOI: https://doi.org/10.1016/j.bioorg.2021.105336.
Gregorić, T.; Sedić, M.; Grbčić, P.; Paravić, A. T.; Pavelić, S. K.; Cetina, M.; Vianello, R.; Raić-Malić, S. Eur. J. Med. Chem. 2017, 125, 1247-1267. DOI: https://doi.org/10.1016/j.ejmech.2016.11.028.
Hossam, M.; Lasheen, D. S.; Ismail, N. S.; Esmat, A.; Mansour, A. M.; Singab, A. N. B.; Abouzid, K. A. Eur. J. Med. Chem. 2018, 144, 330-348. DOI: https://doi.org/10.1016/j.ejmech.2017.12.022.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J., Gaussian 09, Revision B.01, Gaussian Inc., Wallingford CT, 2009.
Yılmaz, A. Ş.; Kaçan, M. Tetrahedron. 2017, 73, 4509-4512. DOI: https://doi.org/10.1016/j.tet.2017.05.072.
Daina, A.; Michielin, O.; Zoete, V. Sci Rep. 2017, 7, 42717. DOI: https://doi.org/10.1038/srep42717.
Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. Bioinformatics. 2018, 35, 1067-1069. DOI: https://doi.org/10.1093/bioinformatics/bty707.
Qu, R.; Zhang, X.; Zhang, Q.; Yang, X.; Wang, Z.; Wang, L. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 81, 261-269. DOI: https://doi.org/10.1016/j.saa.2011.06.008.
Manikandan, D.; Swaminathan, J.; Tagore, S. S.; Gomathi, S.; Sabarinathan, N.; Ramalingam, M.; Balasubramani, K.; Sethuraman, V. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118484. DOI: https://doi.org/10.1016/j.saa.2020.118484.
Foresman, J.; Frish, E. in: Exploring Chemistry with Electronic Structure Methods, Gaussian Inc., Pittsburg, USA, 1996.
Sayin, K.; Karakaş, D. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 144, 176-182. DOI: https://doi.org/10.1016/j.saa.2015.02.086.
Uluçam, G.; Okan, Ş. E.; Aktaş, Ş.; Yentürk, B. J. Mol. Struct. 2021, 1230, 129941. DOI: https://doi.org/10.1016/j.molstruc.2021.129941.
Dennington, R.; Keith, T.; Millam, J. Gauss View, Version 5. Semichem Inc., Shawnee Mission, 2009.
Zhen, Y.; Shan, X.; Li, Y.; Lin, Z.; Zhang, L.; Lai, C.; Qin, F. Phytomed. Plus. 2022, 100244. DOI: https://doi.org/10.1016/j.phyplu.2022.100244.
Yadav, V.; Krishnan, A.; Baig, M. S.; Majeed, M.; Nayak, M.; Vohora, D. Biophys. Chem. 2022, 285. DOI: https://doi.org/10.1016/j.bpc.2022.106808.
Anju, K.; Shoba, G.; Sumita, A.; Balakumaran, M. D.; Vasanthi, R.; Kumaran, R. Spectrochim. Acta, Part A. 2021, 258, 119814. DOI: https://doi.org/10.1016/j.saa.2021.119814.
Crampon, K.; Giorkallos, A.; Deldossi, M.; Baud, S.; Steffenel, L. A. Drug Discovery Today. 2021, 151-164. DOI: https://doi.org/10.1016/j.drudis.2021.09.007.
Trott, O.; Olson, A. J. J. Comput. Chem. 2010, 31, 455-461. DOI: https://doi.org/10.1002/jcc.21334.
Morris, G. M.; Huey, R.; Lindstrom, W.; Sanner, M. F.; Belew, R. K.; Goodsell, D. S.; Olson, A. J. J. Comput. Chem. 2009, 30, 2785-2791. DOI: https://doi.org/10.1002/jcc.21256.
Fatima, A.; Khanum, G.; Sharma, A.; Verma, I.; Arora, H.; Siddiqui, N.; Javed, S. Polycycl. Aromat. Compd. 2023, 43, 1263-1287. DOI: https://doi.org/10.1080/10406638.2022.2026989.
Yavuz, S. Ç.; Akkoç, S.; Tüzün, B.; Şahin, O.; Saripinar, E. Synth. Commun. 2021, 51, 2135-2159. DOI: https://doi.org/10.1080/00397911.2021.1922920.
Xavier, T.; Kenny, P. T.; Manimaran, D.; Joe, I. H. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 145, 523-530. DOI: https://doi.org/10.1016/j.saa.2015.02.087.
Suresh, D.; Amalanathan, M.; Joe, I. H.; Jothy, V. B.; Diao, Y.-P. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 591-603. DOI: https://doi.org/10.1016/j.saa.2014.03.043.
Devi, K.S.; Subramani, P.; Parthiban, S.; Sundaraganesan, N. J. Mol. Struct. 2020, 1203, 127403. DOI: https://doi.org/10.1016/j.molstruc.2019.127403.
Adwin Jose, P.; Sankarganesh, M.; Dhaveethu Raja, J.; Senthilkumar, G. S.; Nandini Asha, R.; Raja, S. J.; Sheela, C. D. J. Biomol. Struct. Dyn. 2021, 21, 10715–10729. DOI: https://doi.org/10.1080/07391102.2021.1947382.
Rodriguez, A. C.; Park, H.-W.; Mao, C.; Beese, L. S. J. Mol. Biol. 2000, 299, 447-462. DOI: https://doi.org/10.1006/jmbi.2000.3728.
Williams, R. S.; Green, R.; Glover, J. Nat. Struct. Biol. 2001, 8, 838-842. DOI: https://doi.org/10.1038/nsb1001-838.
Rouvinen, J.; Rautiainen, J.; Virtanen, T.; Zeiler, T.; Kauppinen, J.; Taivainen, A.; Mäntyjärvi, R. J. Biol. Chem. 1999, 274, 2337-2343. DOI: https://doi.org/10.1074/jbc.274.4.2337.
Okamoto, K.; Ikemori-Kawada, M.; Jestel, A.; von König, K.; Funahashi, Y.; Matsushima, T.; Tsuruoka, A.; Inoue, A.; Matsui, J. ACS Med. Chem. Lett. 2015, 6, 89-94. DOI: https://doi.org/10.1021/ml500394m.
Yun, C.-H.; Boggon, T. J.; Li, Y.; Woo, M. S.; Greulich, H.; Meyerson, M.; Eck, M. J. Cancer cell. 2007, 11, 217-227. DOI: https://doi.org/10.1016/j.ccr.2006.12.017.
Brough, P. A.; Aherne, W.; Barril, X.; Borgognoni, J.; Boxall, K.; Cansfield, J. E.; Cheung, K.-M. J.; Collins, I.; Davies, N. G.; Drysdale, M. J. J. Med. Chem. 2008, 51, 196-218. DOI: https://doi.org/10.1021/jm701018h.
Masand, V. H.; Rastija, V. Chemom. Intell. Lab. Syst. 2017, 169, 12-18. DOI: https://doi.org/10.1016/j.chemolab.2017.08.003.
DeLano, W. L. http://www.pymol.org, 2002.
Pettersen, E. F.; Goddard, T. D.; Huang, C. C.; Couch, G. S.; Greenblatt, D. M.; Meng, E. C.; Ferrin, T. E. J. Comput. Chem. 2004, 25, 1605-1612. DOI: https://doi.org/10.1002/jcc.20084.
Laskowski, R. A.; Swindells, M. B. J. Chem. Inf. Model. 2011, 51, 2778-2786 DOI: https://doi.org/10.1021/ci200227u.
Lipinski, C. A. Drug. Discov. Today. Technol. 2004, 1, 337-41. DOI: https://doi.org/10.1016/j.ddtec.2004.11.007.
Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P. W.; Tang, Y. J. Chem. Inf. Model. 2012, 52, 3099-3105. DOI: https://doi.org/10.1021/ci300367a.
Saravanan, R.; Seshadri, S.; Gunasekaran, S.; Mendoza-Meroño, R.; García-Granda, S. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 139, 321-328. DOI: https://doi.org/10.1016/j.saa.2014.12.026.
Demircioğlu, Z.; Kaştaş, Ç. A.; Büyükgüngör, O. 2015, 1091, 183-195. DOI: https://doi.org/10.1016/j.molstruc.2015.02.076.
Obu, Q. S.; Louis, H.; Odey, J. O.; Eko, I. J.; Abdullahi, S.; Ntui, T. N.; Offiong, O. E. J. Mol. Struct. 2021, 1244, 130880. DOI: https://doi.org/10.1016/j.molstruc.2021.130880.
Mumit, M. A.; Pal, T. K.; Alam, M. A.; Islam, M. A.-A.-A.-A.; Paul, S.; Sheikh, M. C. J. Mol. Struct. 2020, 1220, 128715. DOI: https://doi.org/10.1016/j.molstruc.2020.128715.
Ouaket, A.; Chraka, A.; Raissouni, I.; El Amrani, M. A.; Berrada, M.; Knouzi, N. J. Mol. Struct. 2022, 1259, 132729. DOI: https://doi.org/10.1016/j.molstruc.2022.132729.
Abdou, A.; Omran, O. A.; Nafady, A.; Antipin, I. S. Arabian J. Chem. 2022, 15, 103656. DOI: https://doi.org/10.1016/j.arabjc.2021.103656.
Ulucam, G.; Yenturk, B.; Okan, S. E.; Aktas, S. Chem. Pap. 2020, 74, 1881-1889. DOI: https://doi.org/10.1007/s11696-019-01037-9.
Almeida, M. O.; Barros, D. A. S.; Araujo, S. C.; Faria, S.; Maltarollo, V. G.; Honorio, K. M. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 184, 169-176. DOI: https://doi.org/10.1016/j.saa.2017.04.070.
Boshaala, A.; Said, M. A.; Assirey, E. A.; Alborki, Z. S.; AlObaid, A. A.; Zarrouk, A.; Warad, I. J. Mol. Struct. 2021, 1238, 130461. DOI: https://doi.org/10.1016/j.molstruc.2021.130461.
Kargar, H.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Munawar, K. S.; Ashfaq, M.; Tahir, M. N. J. Mol. Struct. 2022, 1250, 131691. DOI: https://doi.org/10.1016/j.molstruc.2021.131691.
Anwer, K. E.; Sayed, G. H.; Ramadan, R. M. J. Mol. Struct. 2022, 1256, 132513. DOI: https://doi.org/10.1016/j.molstruc.2022.132513.
Śmiszek-Lindert, W. E.; Chełmecka, E.; Lindert, O.; Dudzińska, A.; Kaczmarczyk-Sedlak, I. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2018, 201, 328-338. DOI: https://doi.org/10.1016/j.saa.2018.05.021.
Umar, Y. J. Mol. Struct. 2022, 133230. DOI: https://doi.org/10.1016/j.molstruc.2022.133230.
Unsalan, O.; Szolnoki, B.; Toldy, A.; Marosi, G. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 98, 110-115. DOI: https://doi.org/10.1016/j.saa.2012.08.050.
Uluçam, G.; Bagcı, U.; Şuekinci Yılmaz, A.; Yentürk, B. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 279, 121429. DOI: https://doi.org/10.1016/j.saa.2022.121429.
Mary, Y. S.; Mary, Y. S.; Resmi, K.; Kumar, V. S.; Thomas, R.; Sureshkumar, B. Heliyon. 2019, 5, e02825. DOI: https://doi.org/10.1016/j.heliyon.2019.e02825.
Daina, A.; Michielin, O.; Zoete, V. J. Chem. Inf. Model. 2014, 54, 3284-3301. DOI: https://doi.org/10.1021/ci500467k.
Daina, A.; Zoete, V. Chem. Med. Chem. 2016, 11, 1117-21. DOI: https://doi.org/10.1002/cmdc.201600182.
Walters, W. P.; Murcko, M. A. Adv. Drug Delivery Rev. 2002, 54, 255-71. DOI: https://doi.org/10.1016/S0169-409X(02)00003-0.

Downloads
Published
Issue
Section
License
Copyright (c) 2024 Gühergül Uluçam

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









