Standardization of Phenols and Flavonoids Extraction from Opuntia ficus-indica Mill Cv. Atlixco and Identification by Mass Spectrometry
DOI:
https://doi.org/10.29356/jmcs.v64i4.1203Palabras clave:
TLC, acidic hydrolysis, chromatography, cactus stemResumen
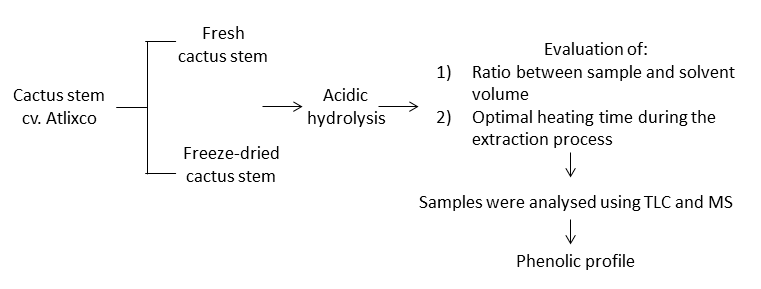
Abstract. The cactus stem of Atlixco cultivar is consumed for its pharmacological properties and it is marketed not only in Mexico but also in other countries. Despite this, its complete phenolic profile is not known which are recognised as powerful antioxidants. This work aimed to develop a standardised methodology for optimise the conditions for phenolic compounds extraction in the cactus stem of Atlixco cultivar and to identify these compounds using mass spectrometry (MS). Results indicated the following: a) it is recommended to use one unit of freeze-dried cactus stem for every 50 units of solvent. Subsequently, the blend should be eluted with water/methanol/acetonitrile in a ratio of 25:25:50 by the column chromatography technique; b) samples maintained in a reflux system with an acidic medium after two hours of heating at 65°C showed the greatest amount of phenolic compounds by MS, and c) trans-caffeic acid, ferulic acid, kaempferol, quercetin, and isorhamnetin were identified. In conclusion, only some of the phenolic compounds identified in this work had been reported in other cactus stem cultivars.
Resumen. El nopal del cultivar Atlixco se consume por sus propiedades farmacológicas y se comercializa no solo en México sino también en otros países. A pesar de esto, se desconoce su perfil de compuestos fenólicos, los cuales son reconocidos como potentes antioxidantes. Este trabajo tuvo como objetivo desarrollar una metodología estandarizada para optimizar las condiciones para la extracción de compuestos fenólicos en el nopal del cultivar Atlixco e identificar estos compuestos usando espectrometría de masas (EM). Los resultados indicaron lo siguiente: a) se recomienda usar una unidad de nopal liofilizado por cada 50 unidades de disolvente. Después la mezcla deberá ser eluida con agua/metanol/acetonitrilo en una proporción de 25:25:50 por la técnica de cromatografía en columna, b) las muestras mantenidas en un medio ácido después de dos horas de calentamiento a 65°C mostraron el mayor número de compuestos fenólicos por EM, y c) se identificaron el ácido trans-cafeico, ácido ferúlico, campferol, quercetina e isoramnetina. En conclusión, solo algunos de los compuestos fenólicos identificados en este trabajo han sido reportados en otros cultivares de nopal.
Descargas
Citas
https://www.gob.mx/siap/documentos/siacon-ng-16143, accessed in July 2019
Sáenz, C.; Berger, H.; Corrales, G.J.; Galletti, L.; García, C.V.; Higuera, I.; Mondragón, C.; Rodríguez, F. A.; Sepúlveda, E.; Varnero, M.T. Boletín de Servicios Agrícolas de la FAO, Paper 162. Rome, Italy. 2006.
Maki-Díaz, G.; Peña-Valdivia, C.B.; García-Nava, R.; Arévalo-Galarza, M.L.; Calderón-Zavala, G.; Anaya-Rosales, S. Agrociencia. 2015, 49(1), 31–51.
http://www.economia-nmx.gob.mx/normas/nmx/2007/nmx-ff-068-scfi-2006.pdf, accessed in November 2019.
Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.W.; De León-Rodríguez, A.; Fomsgaard, I.S.; Barba de la Rosa A.P. J. Food Compos. Anal. 2010, 23(6), 525–32. DOI:10.1016/j.jfca.2009.12.003. DOI: https://doi.org/10.1016/j.jfca.2009.12.003
Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa AP. J. Food Compos. Anal. 2015, 43,119–30. DOI.org/10.1016/j.jfca.2015.04.016 0889-1575/ß DOI: https://doi.org/10.1016/j.jfca.2015.04.016
Ventura-Aguilar, R.I.; Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera F. J. Sci Food Agric. 2017, 97(15), 5065–73. DOI: 10.1002/jsfa.8493. DOI: https://doi.org/10.1002/jsfa.8493
Pérez, L.; Tejera, F.; Darias, J; Rodríguez, E.M.; Díaz C. Food Chem. 2015, 188, 393–8. DOI.org/10.1016/j.foodchem.2015.05.011 DOI: https://doi.org/10.1016/j.foodchem.2015.05.011
Aldred, EM.; Buck, C.; Vall, K. in: Pharmacology: A Handbook for Complementary Healthcare Professionals, Aldred, EM.; Buck, C.; Vall K, editors, Churchill Livingstone, UK, 2009, 149–66. DOI: https://doi.org/10.1016/B978-0-443-06898-0.00021-9
Rice-Evans, C.A.; Miller, N.J; Paganga G. Free Radic Biol Med. 1996, 20(7), 933–56. DOI: https://doi.org/10.1016/0891-5849(95)02227-9
Kuti, JO. Food Chem. 2004, 85(4), 527–33. DOI:10.1016/S0308-8146(03)00184-5.. DOI: https://doi.org/10.1016/S0308-8146(03)00184-5
Watson, R.; Preedy V. Academic Press Ed. 2009.
Martínez-Flórez, S.; González-Gallego, J.; Culebras, J.M.; Tuñón MJ. Nutr Hosp. 2002, 17(6), 271–8.
Routray, W.; Orsat, V. Food Bioprocess Technol. 2012, 5(2), 409–24. DOI: 10.1007/s11947-011-0573-z DOI: https://doi.org/10.1007/s11947-011-0573-z
Ventura-Aguilar, R.I.; Rivera-Cabrera, F.; Méndez-Iturbide, D.; Pelayo-Zaldívar, C.; Bosquez-Molina, E. Int J Food Sci Technol. 2013, 48(12), 2603–12. DOI:10.1111/ijfs.12256 DOI: https://doi.org/10.1111/ijfs.12256
Gupta, R.S.; Sharma, R.; Sharma, A.; Chaudhudery, R.; Bhatnager, A.K.; Dobhal, M.P.; Joshi, Y.C.; Sharma, MC. Pharm Biol. 2002, 40(6), 411–5. DOI.org/10.1076/phbi.406.411.8437 DOI: https://doi.org/10.1076/phbi.40.6.411.8437
Stintzing, F.C.; Carle, R. Mol Nutr Food Res. 2005, 49(2),175–94. DOI: 10.1002/mnfr.200400071 DOI: https://doi.org/10.1002/mnfr.200400071
Antunes-Ricardo, M.; Moreno-García, B.; Gutiérrez-Uribe, J.; Aráiz-Hernández, D.; Serna-Saldivar, S.; Alvarez, M. Plant Foods Hum Nutr. 2014, 69(4), 331–6. DOI.org/ 10.1007/s11130-014-0438-5 DOI: https://doi.org/10.1007/s11130-014-0438-5
Moussa-Ayoub, T.E; Abd El-Hady, E.S.A; Omran, H.T; El-Samahy, S.K; Kroh L.W; Rohn S. Food Res Int. 2014, 64, 864–72. DOI: https://doi.org/10.1016/j.foodres.2014.08.021
Mustafa, R.A.; Hamid, A.A.; Mohamed, S.; Bakar, FA. J Food Sci. 2010, 75(1), 28–35. DOI: 10.1111/j.1750-3841.2009.01401.x DOI: https://doi.org/10.1111/j.1750-3841.2009.01401.x
Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, BD. J. Agric Food Chem. 1998, 46(10), 4113–7. DOI: https://doi.org/10.1021/jf9801973
Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Food Chem. 2013, 139(1–4),796–803. DOI: https://doi.org/10.1016/j.foodchem.2013.01.054
Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.F.; Lacroix M. Crit Rev Food Sci Nutr. 2010, 50(9), 872–88. DOI: 10.1080/10408390903042069. DOI: https://doi.org/10.1080/10408390903042069
Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, JR. Critical Reviews in Biotechnology. 2011, 31(3), 227–49. DOI: 10.3109/07388551.2010.513677 DOI: https://doi.org/10.3109/07388551.2010.513677
Petyuk, V.A.; Jaitly, N.; Moore, R.J.; Ding, J.; Metz, T.O.; Tang, K.; Monroe, M.E.; Tolmachev, A.V.; Adkins, J.N.; Belov, M.E.; Dabney, A.R.; Qian, W.J.; Camp, D.G.; Smith, R.D. Anal Chem. 2008, 80(3), 693–706. DOI: 10.1021/ac701863d DOI: https://doi.org/10.1021/ac701863d
Trabelsi, N.; Megdiche, W.; Ksouri, R.; Falleh, H.; Oueslati, S.; Soumaya, B.; Hajlaoui, H.; Abdelly, C. LWT-Food Sci Technol. 2010, 43, 632–9. DOI: 10.1016/j.lwt.2009.11.003 DOI: https://doi.org/10.1016/j.lwt.2009.11.003
March, R.E.; Miao, XS. Int J Mass Spectrom. 2004, 231, 157–67. DOI:10.1016/j.ijms.2003.10.008 DOI: https://doi.org/10.1016/j.ijms.2003.10.008
Santos-Zea, L.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. J. Agric Food Chem. 2011, 59(13), 7054–61. dx.doi.org/10.1021/jf200944y DOI: https://doi.org/10.1021/jf200944y
Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.; Goñi, I. Plant Foods Hum Nutr. 2010, 65(3), 210–6. DOI: 10.1007/s11130-010-0176-2 DOI: https://doi.org/10.1007/s11130-010-0176-2
Biesaga, M. J. Chromatogr. A. 2011, 1218(18), 2505–12. DOI:10.1016/j.chroma.2011.02.059 DOI: https://doi.org/10.1016/j.chroma.2011.02.059
Biesaga, M.; Pyrzy?ska, K. Food Chem. 2013, 136(1), 46–54. http://dx.doi.org/10.1016/j.foodchem.2012.07.095 DOI: https://doi.org/10.1016/j.foodchem.2012.07.095
Nuutila, A.M.; Kammiovirta, K.; Oksman-Caldentey, K.M. Food Chem. 2002, 76(4), 519–25. DOI: https://doi.org/10.1016/S0308-8146(01)00305-3
Sani, I.M.; Iqbal, S.; Chan, K.W.; Ismail M. Molecules. 2012, 17(6), 7584–94. DOI:10.3390/molecules17067584 DOI: https://doi.org/10.3390/molecules17067584
Hertog, M.G.L.; Hollman, P.C.H.; Venema, D.P.J. Agric Food Chem. 1992, 40(9), 1591-1598. DOI: https://doi.org/10.1021/jf00021a023
Maini, S.; Hodgson, H.L.; Krol, E.S. J. Agric Food Chem. 2012, 60(28), 6966–76. dx.doi.org/10.1021/jf3016128 DOI: https://doi.org/10.1021/jf3016128
Makris, D.P.; Rossiter, J.T. Food Chem. 2002, 77(2), 177–85. DOI: https://doi.org/10.1016/S0308-8146(01)00333-8
Zhou, A.; Kikandi, S.; Sadik, OA. Electrochem Commun. 2007, 9(9), 2246–55. DOI:10.1016/j.elecom.2007.06.026 DOI: https://doi.org/10.1016/j.elecom.2007.06.026
Mattila, P.; Hellström, J. J. Food Compos Anal. 2007, 20(3–4), 152–60. doi:10.1016/j.jfca.2006.05.007 DOI: https://doi.org/10.1016/j.jfca.2006.05.007
Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. J. Nutr Biochem. 2002, 13(10), 572–84. DOI: https://doi.org/10.1016/S0955-2863(02)00208-5
Ginestra, G.; Parker, ML.; Bennett, RN.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, CB.; Waldron, K. J. Agric Food Chem. 2009, 57(21), 10323–30. DOI: https://doi.org/10.1021/jf9022096

Descargas
Publicado
Número
Sección
Licencia
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








