Continuous Manufacturing in Pharmaceutical Industry: How it Thrives Green Chemistry Principles
DOI:
https://doi.org/10.29356/jmcs.v69i4.2326Keywords:
Continuous manufacturing, green chemistry, pharmaceutical industry, drug manufacturing, flow processAbstract
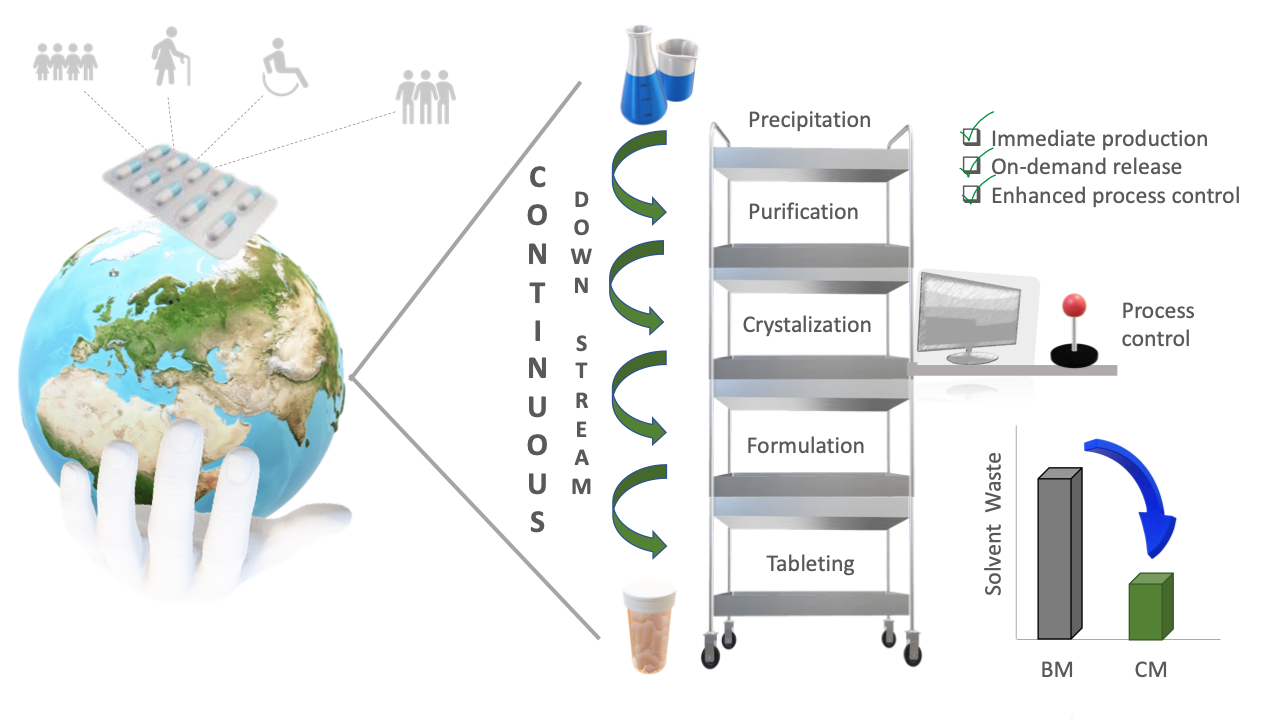
Abstract. Green Chemistry is an approach that looks for a sustainable development, encouraging the industry to migrate towards cleaner and more efficient processes, reducing waste generation, and depleting carbon footprint. Continuous Manufacturing is an advanced production technology that has caught attention during the past decade because of its flexibility, agility and robustness; lately, some regulatory agencies have endorsed its implementation, but there are only a few cases of its actual applications in the manufacturing of drug substances and products. In the current paper, we review some examples available up to date in which continuous processes and green chemistry principles converge in the pharma-chemical & pharmaceutical industries in order to bring elements to understand the future of drug molecules and pharmaceutical products production.
Resumen. La Química Verde es un enfoque que busca un desarrollo sostenible, alentando a la industria a migrar hacia procesos más limpios y eficientes, reduciendo la generación de residuos y la huella de carbono. La Manufactura Continua es una tecnología de producción avanzada que ha llamado la atención durante la última década por su flexibilidad, agilidad y robustez; últimamente, algunas agencias reguladoras han avalado su implantación, pero sólo existen unos pocos casos de sus aplicaciones reales en la fabricación de fármacos y medicamentos. En el presente artículo, repasamos algunos ejemplos disponibles en los que convergen la manufacturas procesos continuos y los principios de la química verde en las industrias farmoquímica y farmacéutica, con el fin de aportar elementos para comprender el futuro de la producción de fármacos y productos farmacéuticos.
Downloads
References
1. Albini, A.; Protti, S., in: Paradigms in Green Chemistry and Technology. Springer, New York-London, 2016. DOI: http://doi.org/10.1007/978-3-319-25895-9_2.
2. Pisano, R. Am. Pharm. Rev. 2020, 23, 1-4.
3. Lee, S. L., O’Connor, T. F., Yang, X., Cruz, C. N., Chatterjee, S., Madurawe, R. D., & … Woodcock, J. J. Pharm. Innovation. 2015, 10, 191–199. DOI: http://doi.org/10.1007/s12247-015-9215-8.
4. Muzzio, F., in: Pharmaceutical Engineering Cycle. Rutgers University. 2021. Rutgers University. Retrieved from: https://youtu.be/ZBbCvE4QYLg?si=VjzCDVdduLpvzD_3
5. Malet-Sanz, L.; Susanne, F. J. Med. Chem. 2012. 55, 4062–4098. DOI: http://doi.org/10.1021/jm2006029.
6. Lawton, S.; Steele, G.; Shering, P.; Zhao, L.; Laird, I.; Ni, X. W. Org. Process Res. Dev. 2009, 13, 1357–1363. DOI: http://doi.org/10.1021/op900237x.
7. Ferguson, S.; Morris, G.; Hao, H.; Barrett, M.; Glennon, B. Chem. Eng. Sci. 2013, 104, 44–54. DOI: http://doi.org/10.1016/j.ces.2013.09.006.
8. Mascia, S.; P.L., H.; Zhang, H.; Lakerveld, R.; Benyahia, B.; Barton, P.; . . . B.L., T. Angew. Chem. Int. Ed. 2013, 52, 12359–12363. DOI: http://doi.org/10.1002/anie.201305429.
9. Gutmann, B.; Cantillo, D.; Kappe, C. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. DOI: http://doi.org/10.1002/anie.201409318.
10. Besenhard, M.; Neugebauer, P.; Scheibelhofer, O.; Khinast, J. Cryst. Growth Des. 2017, 17, 6432-6444. DOI: http://doi.org/10.1021/acs.cgd.7b01096.
11. McLaughlin, A.; Robertson, J.; Ni, X. W. Pharm. Dev. Technol. 2019, 25, 1–28. DOI: https://doi.org/10.1080/10837450.2019.1685543.
12. Aranda, L. A., in: Aplicaciones de la Manufactura Continua y la Química Verde en la producción de Fármacos y Medicamentos. Mstr. D. Thesis, Universidad Autónoma del Estado de México, Toluca, México, 2024.
13. Pájaro, N., Olivero, J. Ciencia e Ingeniería Neogranadina. 2011. 21,169-182.
14. Kulkarni, S.; Rawat, N.; Haghi, A., in: Green chemistry and green engineering: processing, technologies, properties and applications. Apple Academic Press, Palm Bay, 2021.
15. Anastas, P.; Williamson, T. C., Green Chemistry: An Overview. In: Green Chemistry: designing chemistry for the environment, vol. 626. 1–17. Washington: ACS symposium series, Washington, D.C. 1996.
16. Anastas, P.; Warner, J., in: Green Chemistry: Theory and Practice. New York: Oxford University Press, 1998.
17. Cannon, A. S.; Pont, J. L.; Warner, J. C., in: Green Techniques for Organic Synthesis and Medicinal Chemistry. John Wiley & Sons, Ltd. 2012. DOI: http://doi.org/10.1039/b312329d10.1002/9780470711828.ch2.
18. Linthorst, J. A. Found. Chem. 2009, 12, 55–68.
19. Cue, B. W.; Zhang, J. Green Chem. Lett. Rev. 2009, 2, 193–211. DOI: http://doi.org/10.1080/17518250903258150.
20. Dunn, P. J.; Galvin, S.; Hettenbach, K. Green Chem. 2004, 6, 43–48. DOI: http://doi.org/10.1039/b312329d.
21. Mountford, P. G. Green Chem. Pharm. Ind. 2010, 145–160. DOI: http://doi.org/10.1002/9783527629688.ch7.
22. Hansen, K. B.; Hsiao, Y.; Xu, F.; Rivera, N.; Clausen, A.; Kubryk, M.; … Armstrong, J. D. Highly J. Am. Chem. Soc. 2009, 131, 8798–8804. DOI: http//doi.org/10.1021/ja902462q.
23. WuXi AppTech Flow Chemistry Page. https://sta.wuxiapptec.com/services-solutions/drug-substance/enabling-technology-platform/flow-chemistry/, accessed in December 2024.
24. Patheon Continuous Manufacturing Page. https://www.patheon.com/us/en/innovative-solutions/manufacturing.html, accessed in December 2024.
25. De Soete, W.; Dewulf, J.; Cappuyns, P.; Van der Vorst, G.; Heirman, B.; Aelterman, W.; ...; Van Langenhove, H. Green Chem. 2013, 15, 3039-3048.
26. Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. G.; PRISMA Group*, T. Ann. Intern. Med. 2009, 151, 264-269. DOI: doi:10.7326/0003-4819-151-4-200908180-00135.
27. Badman, C.; Cooney, C. L.; Haslam, R. T.; Florence, A.; Konstantinov, K.; Krumme, M.; … Baddour, R. F. J. Pharm. Sci. 2019, 108, 3521-3523. DOI: http://doi.org/10.1016/j.xphs.2019.07.016.
28. Food and Drug Administration. Quality Considerations for Continuous Manufacturing Guidance for Industry. 2019. Retrieved from https://www.gmp-compliance.org/files/guidemgr/UCM632033.pdf.
29. Khinast, J.; Kleinebudde, P.; Rantanen, J. in: Continuous Manufacturing of Pharmaceuticals. John Wiley & Sons Ltd., Hoboken, 2017.
30. Food and Drug Administration. Pharmaceutical cGMPs for the 21st Century: A Risk-Based Approach. 2004. Retrieved from https://www.fda.gov/media/77391/download.
31. Berridge, J. ICH Q8: Pharmaceutical Development. Pharmaceutical Quality Forum, November 2004. Amsterdam-The Netherlands. 2004. Retrieved from http://www.nihs.go.jp/drug/PhForum/documents041122/Berridge041122.pdf.
32. International Conference on Harmonization. Pharmaceutical Development Guideline Q8(R2). 2009. Retrieved from: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf.
33. International Conference on Harmonization. Quality Risk Management Q9(R1). 2023. Retrieved from: https://database.ich.org/sites/default/files/ICH_Q9%28R1%29_Guideline_Step4_2023_0126_0.pdf.
34. Food and Drug Administration. Guidance for Industry — PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. Rockville, MD, EUA. 2004. Retrieved from https://www.fda.gov/media/71012/download.
35. International Conference on Harmonization. Continuous Manufacturing of Drug Substances and Drug Products Guideline Q13. 2022. Retrieved from: https://www.ich.org. Retrieved from https://database.ich.org/sites/default/files/ICH_Q13_Step4_Guideline_2022_1116.pdf.
36. Shores, B. T.; Sieg, P. E.; Nicosia, A. T.; Hu, C.; Born, S. C.; Shvedova, K.; Mascia, S. Org. Process Res. Dev. 2020. 24, 10, 1996–2003. DOI: http://doi.org/10.1021/acs.oprd.0c00092.
37. Johnson&Johnson Home Page. https://innovativemedicine.jnj.com/, accessed in December 2024.
38. Adamo, A.; Beingessner, R. L.; Behnam, M.; Chen, J.; Jamison, T. F.; Jensen, K. F.; … Zhang, P. Science. 2016, 352, 61–67. DOI: http://doi.org/10.1126/science.aaf1337.
39. Capellades, G.; Neurohr, C.; Briggs, N.; Rapp, K.; Hammersmith, G.; Brancazio, D.; … Myerson, A. S. Org. Process Res. Dev. 2021, 25, 1534–1546. DOI: http://doi.org/10.1039/b312329d10.1021/acs.oprd.1c00117.
40. Zhang, P.; Weeranoppanant, N.; Thomas, D. A.; Tahara, K.; Stelzer, T.; Russell, M. G.; …; Adamo, A. Chem. – Eur. J. 2018, 24, 2776–2784. DOI: http://doi.org/10.1002/chem.20.
41. Muzzio, F., in: Continuous manufacturing of tablets and capsules - The Emerging Paradigm. Oral Presentation. Rutgers University. 2016. Retrieved from USP.org: https://www.usp.org/sites/default/files/usp/document/our-work/research-innovation/research-innovation-muzzio-presentation.pdf.
42. Rogers, L.; Jensen, K. F. Green Chem. 2019, 13, 3481-3498. DOI: http://doi.org/10.1039/c9gc00773c.
43. Falß, S.; Kloye, N.; Holtkamp, M.; Prokofyeva, A.; Bieringer, T.; Kockmann, N. Handb. Green Chem. 2019. 153–190. DOI: http://doi.org/10.1002/9783527628698.hgc139.
44. Martin, B.; Lehmann, H.; Yang, H.; Chen, L.; Tian, X.; Polenk, J.; Schenkel, B. Curr. Opin. Green Sustainable Chem. 2018, 11, 27-33. DOI: http://doi.org/10.1016/j.cogsc.2018.03.005.
45. Domokos, A.; Nagy, B.; Gyürkés, M.; Farkas, A.; Tacsi, K.; Pataki, H.; Kristóf Nagy, Z. Int. J. Pharm. 2020, 581, 1-11. DOI: http://doi.org/10.1016/j.ijpharm.2020.119297.
46. Bennett, J. A.; Campbell, Z. S.; Abolhasani, M. Curr. Opin. Chem. Eng. 2019, 26, 9–19. DOI: http://doi.org/10.1016/j.coche.2019.07.007.
47. May, S. A. J. Flow Chem. 2017, 7, 137–145. DOI: http://doi.org/10.1556/1846.2017.00029.
48. Cole, K. P.; Groh, J. M.; Johnson, M. D.; Burcham, C. L.; Campbell, B. M.; Diseroad, W. D.; Gowran, O. Science. 2017, 356, 1144–1150. DOI: http://doi.org/10.1039/b312329d10.1126/science.aan0745.
49. Pfizer Continuous Manufacturing Press Release Page. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_collaboration_with_gsk_on_next_generation_design_of_portable_continuous_miniature_and_modular_pcmm_oral_solid_dose_development_and_manufacturing_units, accessed in December 2024.
50. European Medicines Agency. Assessment report Daurismo. 2020. Retrieved from: https://www.ema.europa.eu/en/documents/assessment-report/daurismo-epar-public-assessment-report_en.pdf.
51. Phuong, J. M.; Penm, J.; Chaar, B.; Oldfield, L. D.; Moles, R. PLOS ONE. 2019, 14, e0215837. DOI: https://doi.org/10.1371/journal.pone.0215837.
52. Armstrong, C. M. Org. Process Res. Dev. 2021, 25, 1524-1533. DOI: http://doi.org/10.1021/acs.oprd.1c00118.
53. Schaber, S. D.; Gerogiorgis, D. I.; Ramachandran, R.; Evans, J. M.; Barton, P. I.; Trout, B. L. Ind. Eng. Chem. Res. 2011, 50, 10083–10092. DOI: http://doi.org/10.1021/ie2006752.
54. World Health Organization. 19th WHO Model List of Essential Medicines. 2015. Retrieved from http://www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf.
55. Rogers, L.; Briggs, N.; Achermann, R.; Adamo, A.; Azad, M.; Brancazio, D.; …; Jensen, K. F. Org. Process Res. Dev. 2020, 24, 2183–2196. DOI: http://doi.org/10.1021/acs.oprd.0c00208.
56. Harding, M. J.; Brady, S.; O’Connor, H.; Lopez-Rodriguez, R.; Edwards, M. D.; Tracy, S.; … Ferguson, S. React. Chem. Eng. 2020, 5, 728–735. DOI: http://doi.org/10.1039/c9re00408d.
57. Gorak, A.; Sorensen, E., in: Distillation: Fundamentals and Principles. Academic Press, Amsterdam, 2014.
58. Azad, M. A.; Osorio, J. G.; Brancazio, D.; Hammersmith, G.; Klee, D. M.; Rapp, K.; Myerson, A. Int. J. Pharm. 2018, 539, 157–164. DOI: http://doi.org/10.1016/j.ijpharm.2018.01.027.
59. Food and Drug Administration. FDA’s Strategic Plan for Preventing and Mitigating Drug Shortages. 2013. Retrieved from https://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM372566.pdf.

Downloads
Published
Issue
Section
License
Copyright (c) 2025 Luis Alberto Aranda-Hernandez, Mariana Ortiz-Reynoso, María Magdalena García-Fabila, Alfonso Durán

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









