A Facile One-pot Four Component Synthesis of Symmetric 1,4-Dihydropyridine Derivatives using CaFe₂O₂ NPs as Heterogeneous Catalyst under Ultrasound Irradiation and Theoretical Studies as Potential Digestive Enzyme Inhibitors
DOI:
https://doi.org/10.29356/jmcs.v69i3.2193Keywords:
Hantzsch reaction, transesterification, 1,4-Dihydropyridine, ultrasound, heterogeneous catalyst, nano-particles, DFT method, molecular docking, ADMETAbstract
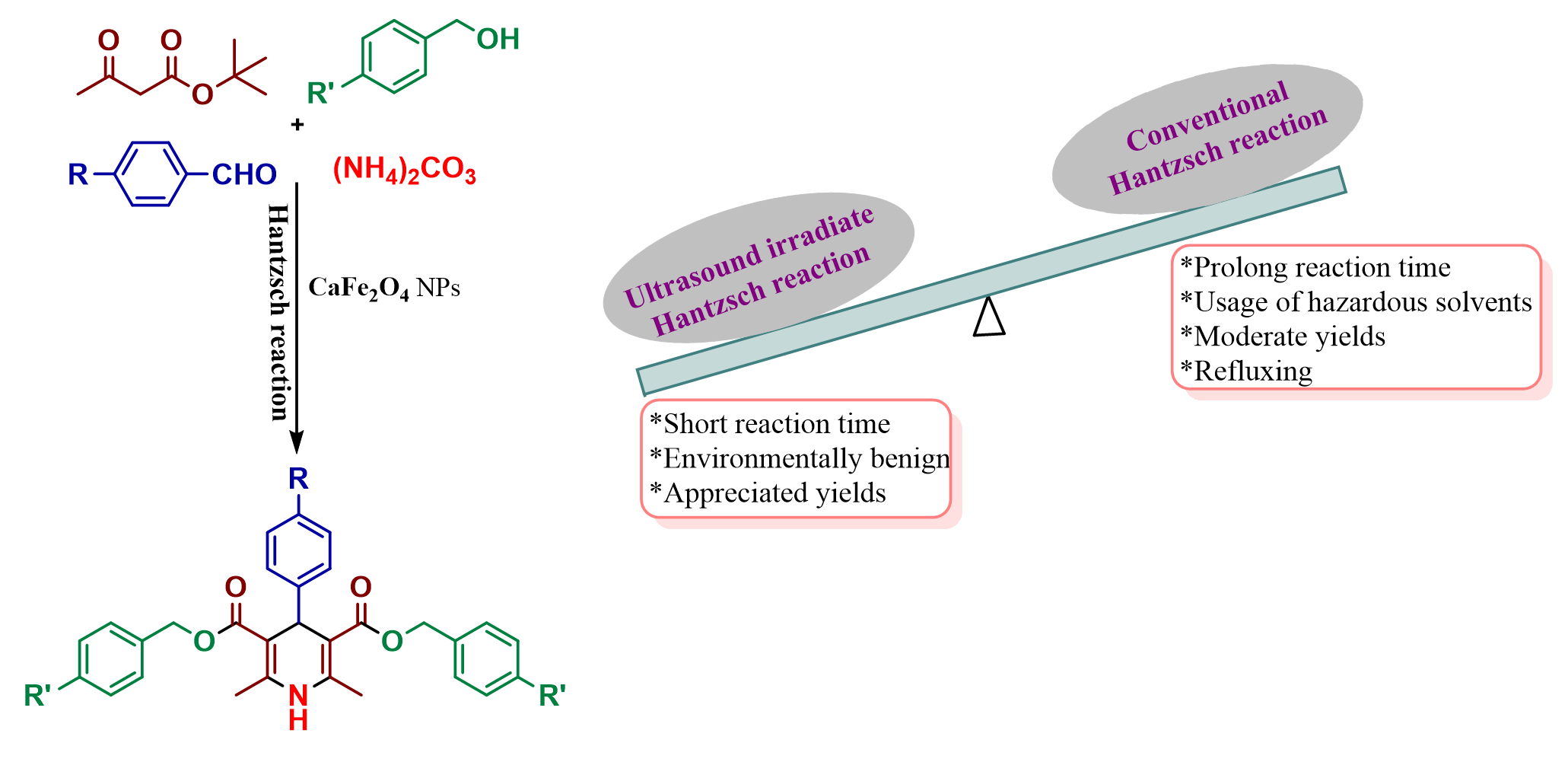
Abstract. A fast and enormously efficient new-flanged symmetric 1,4-dihydropyridine analogs were synthesized by the one-pot four-component condensation reaction of substituted arylaldehyde, tert-butyl β-ketoester, (NH4)2CO3, and benzyl alcohol with p-substitution via transesterification followed by Hantzsch ester synthesis using robust and recyclable CaFe2O4 NPs as heterogeneous catalyst under ultrasonic irradiation. The synthesized compounds were well characterized, and the desired derivatives were studied for the quantum chemical computations using density functional theory (DFT) with Spartan software. On the other hand, the molecular docking experience of all compounds was performed to examine their efficacy against digestive enzymes α-amylase, pepsin, and trypsin and observed that the 1,4-dihydropyridine derivatives could be used as effective digestive enzyme inhibitors.
Resumen. Se sintetizaron de forma rápida y altamente eficiente nuevos análogos simétricos de 1,4-dihidropiridina mediante una reacción de condensación en un solo recipiente y en cuatro componentes, utilizando arilaldehídos sustituidos, β-cetoéster tert-butilo, (NH₄)₂CO₃ y alcohol bencílico con sustitución en posición para a través de una transesterificación, seguida de una síntesis tipo éster de Hantzsch, empleando nanopartículas de CaFe₂O₄ robustas y reciclables como catalizador heterogéneo bajo irradiación ultrasónica. Los compuestos sintetizados fueron debidamente caracterizados, y los derivados obtenidos se estudiaron mediante cálculos químicos cuánticos usando la teoría del funcional de la densidad (DFT) con el software Spartan. Por otro lado, se realizó un estudio de acoplamiento molecular (docking) de todos los compuestos para evaluar su eficacia contra enzimas digestivas como α-amilasa, pepsina y tripsina, observándose que los derivados de 1,4-dihidropiridina podrían emplearse como inhibidores eficaces de enzimas digestivas.
Downloads
References
Saranya, S.; Radhika, S.; Abdulla, C. M. A.; Anilkumar, G. J. Heterocyclic Chem. 2021, 1-11.
Draye, M.; Chatel, G.; Duwald, R. Pharmaceuticals. 2020, 13, 1-35. DOI: https://doi.org/10.3390/ph13020023
Chandan, R.; Mehta, S.; Banerjee, R. Biomater. Sci. Eng. 2020, 6, 4731–4747. DOI: https://doi.org/10.1021/acsbiomaterials.9b01979
Deng, Q.; Mi, J.; Dong, J.; Chen, Y.; Chen, L.; He, J.; Zhou, J. ACS Nano, 2023, 17, 263-274. DOI: https://doi.org/10.1021/acsnano.2c07300
Debnath, K.; Sarkar, A. K.; Jana, N. R.; Jana, N. R. Acc. Mater. Res. 2022, 3, 54-66. DOI: https://doi.org/10.1021/accountsmr.1c00193
Kashkooli, F. M.; Jakhmola, A.; Hornsby, T. K.; Tavakkoli, J.; Kolios, M. C. J. Control. Release. 2023, 355, 552-578. DOI: https://doi.org/10.1016/j.jconrel.2023.02.009
Weissler, A. J. Acoust. Soc. Am. 1953, 25, 651–657. DOI: https://doi.org/10.1121/1.1907158
Rad, T. S.; Ansarian, Z.; Khataee, A.; Vahid, B.; Doustkhah, E. Sep. Purif. Technol. 2021, 256, 117811. DOI: https://doi.org/10.1016/j.seppur.2020.117811
Cella, R.; Stefani, H. A. Tetrahedron. 2009, 65, 2619–2641. DOI: https://doi.org/10.1016/j.tet.2008.12.027
Stefani, H. A.; Cella, R., in: Encyclopedia of Physical Organic Chemistry; John Wiley & Sons, 2017, 1–25.
Saini, K. K.; Rani, R.; Muskan; Khanna, N.; Mehta, B.; Kumar, R. Curr. Org. Chem. 2023, 27, 119-129. DOI: https://doi.org/10.2174/1385272827666230403112419
Hantzsch, A. Chem. Ber. 1881, 14, 1637-1638. DOI: https://doi.org/10.1002/cber.18810140214
Sohal, H. S. Mater. Today: Proc. 2022, 48, 1163-1170. DOI: https://doi.org/10.1016/j.matpr.2021.08.209
Dharma Rao, G. B.; Nagakalyan, S.; Prasad, G. K. RSC Adv. 2017, 7, 3611-3616. DOI: https://doi.org/10.1039/C6RA26664A
Jadeja, K. F.; Joshi, H. S. Anal. Chem. Lett. 2022, 12, 371-379. DOI: https://doi.org/10.1080/22297928.2022.2036233
Xia, Y. N.; Yang, P. D.; Sun, Y. G.; Wu, Y. Y.; Mayers, B.; Gates, B.; Yin, Y. D.; Kin, F.; Yan, H. Q. Adv. Mater. 2003, 15, 353. DOI: https://doi.org/10.1002/adma.200390087
Chan, S. S.; Low, S. S.; Chew, K. W.; Ling, T. C.; Rinklebe, J.; Juan, J. C.; Ng, E. P.; Show, P. L. Environ. Res. 2022, 212, 113140. DOI: https://doi.org/10.1016/j.envres.2022.113140
Chandel, M.; Ghosh, B. K.; Moitra, D.; Patra, M. K.; Vadera, S. R.; Ghosh, N. N. J. Nanosci. Nanotechnol. 2018, 18, 2481-2492. DOI: https://doi.org/10.1166/jnn.2018.14345
Chavan, P.; Bangale, S.; Pansare, D.; Shelke, R.; Jadhav, S.; Tupare, S.; Kamble, D.; Rai, M. J. Heterocyclic Chem. 2020, 1-8.
Shearer, C.; Desaunay, O.; Zorc, S.; Richaud, A. D.; Samanta, S. S.; Jeedimalla, N.; Roche, S. P. Tetrahedron. 2019, 75, 130606. DOI: https://doi.org/10.1016/j.tet.2019.130606
Anjaneyulu, B.; Dharma Rao, G. B.; Sharma, N.; Singh, M. P. Results Chem. 2021, 3, 100202. DOI: https://doi.org/10.1016/j.rechem.2021.100202
Anjaneyulu, B.; Dharma Rao, G. B.; Bajaj, T. Results Chem. 2021, 100093. DOI: https://doi.org/10.1016/j.rechem.2020.100093
Anjaneyulu, B.; Dharma Rao, G. B.; Nancy; Nagakalyan, S. J. Saudi Chem. Soc. 2021, 25, 101394. DOI: https://doi.org/10.1016/j.jscs.2021.101394
Anjaneyulu, B.; Dharma Rao, G. B.; Sharma, N.; Tomar, R.; Singh, L. ChemistrySelect. 2022, 7, e202103910, 1-10.
Anjaneyulu, B.; Dharma, G. B. Lett. Org. Chem. 2023, 20, 568-578.
Anjaneyulu, B.; Arti, S.; Dharma Rao, G. B.; Raza, M. J.; Sharma, N. J. Chem. 2021, 1-12.
Dharma Rao, G. B. J. Heterocyclic Chem. 2018, 55, 2556-2562. DOI: https://doi.org/10.1002/jhet.3309
Dharma Rao, G. B.; Kaushik, M. P. Tetrahedron Lett. 2011, 52, 5104-5106. DOI: https://doi.org/10.1016/j.tetlet.2011.07.108
Witzeman, J. S.; Nottingham, W. D. J. Org. Chem. 1991, 56, 1713. DOI: https://doi.org/10.1021/jo00005a013
Spartan’14, 2014, Wavefunction Inc., Irvine.
Fleming, I., in: Frontier Orbitals and Organic Chemical Reactions, Wiley, London, 1976.
Okulik, N.; Jubert, A. H. J. Mol. Struct. 2004, 682, 55–62. DOI: https://doi.org/10.1016/j.theochem.2004.04.069
Agu, P. C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E. M.; Ofoke, I. H.; Ogbu, C. O.; Ugwuja, E. I.; Aja, P. M. Sci. Rep. 2023, 13, 13398. DOI: https://doi.org/10.1038/s41598-023-40160-2
Meng, X. Y.; Zhang, H. X.; Mezei, M.; Cui, M. Curr. Comput.-Aided Drug Des. 2011, 7, 146-157. DOI: https://doi.org/10.2174/157340911795677602
Ramasubbu, N.; Paloth, V.; Luo, Y.; Brayer, G. D.; Levine, M. J. Acta Cryst. 1996, D52, 435-446. DOI: https://doi.org/10.1107/S0907444995014119
Kesavulu, M. M.; Ramasubramanian, S.; Suguna, K. Biochem. Biophys. Res. Commun. 2005, 331, 1510-1514. DOI: https://doi.org/10.1016/j.bbrc.2005.03.247
Song, H. K.; Suh, S. W. J. Mol. Biol. 1998, 275, 347-363. DOI: https://doi.org/10.1006/jmbi.1997.1469
Trott, O.; Olson, A. J. J. Comput. Chem. 2010, 31, 455-461. DOI: https://doi.org/10.1002/jcc.21334
Daina, A.; Michielin, O.; Zoete, V. Sci. Rep. 2017, 7, 42717. DOI: https://doi.org/10.1038/srep42717
Molinspiration. (n.d.). Molinspiration: Computational chemistry tools for drug discovery.

Downloads
Published
Issue
Section
License
Copyright (c) 2025 Anjaneyulu Bendi, N. Mujafarkani, G. B. Dharma Rao

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








