Antiproliferative and anti-inflammatory acyl glucosyl flavones from the leaves of Bursera copallifera
DOI:
https://doi.org/10.29356/jmcs.v62i4.624Keywords:
Bursera copallifera, Burseraceae, anti-inflammatory activity, antiproliferative activity, acyl glucosyl flavonesAbstract
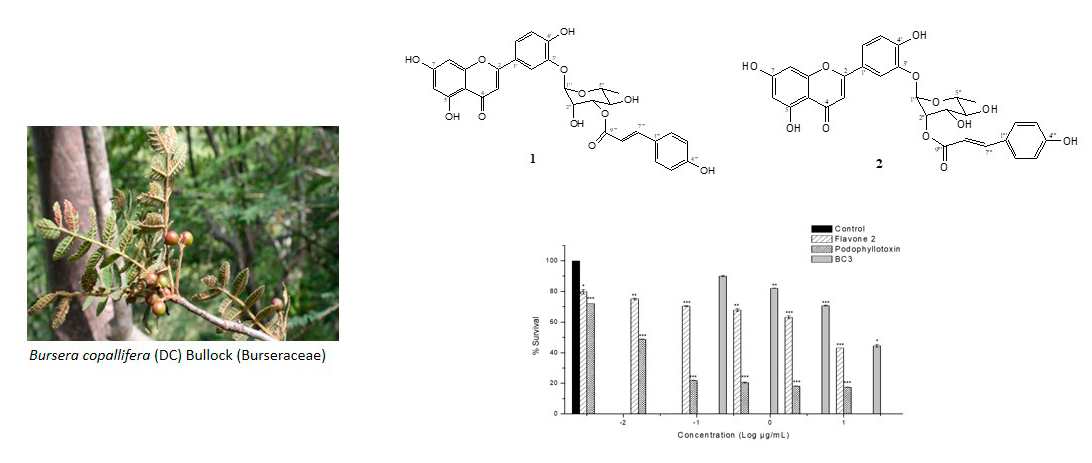
Bursera copallifera(DC) Bullock (Burseraceae) is a plant species used in folk medicine for the treatment of inflammation (bronchitis, cough). The dichloromethane-methanol extract (DML) of B. copallifera leaves was found to possess important antiproliferative and anti-inflammatory activities. In order to further correlate these activities with the phytochemical components, the bioactivity-guided fractionation of the DML extract allowed the isolation of an active fraction which was constituted of five compounds characterized as as luteolin-3'-O-(3''-O-E-p-coumaroyl)-α-L-rhamnopyranoside (1), luteolin-3'-O-(2''-O-E-p-coumaroyl)-α-L-rhamnopyranoside (2), a-amyrin (3), 3-epilupeol (4) and stigmasterol (5). Among the five isolated constituents, the triterpenes 3 and 4, and the sterol 5 have been already well known by their anti-inflammatory properties. Flavone 2 (Luteolin-3'-O-(2''-O-E-p-coumaroyl)-α-L-rhamnopyranoside)was responsible for the antiproliferative activity, showing in vitro cytotoxic activity against MCF-7 human cancer cell line with IC50 value of 13.9 µM. By the other hand flavone (1) exhibited COX-1 inhibition (IC50 = 93 mM). Flavone 1, together with the triterpenes 3 and 4 were the responsible for inhibiting TPA-induced inflammation by inhibit COX-1 enzyme. Therefore, these results support the traditional use of the infusion made with the leaves of B. copallifera as an anti-inflammatory agent.Downloads
References
Maltese, F.; Erkelens, C.; Kooy, F. V. D.; Choi, Y. H.; Verpoorte, R. Food Chem 2009, 116, 575–579. DOI: https://doi.org/10.1016/j.foodchem.2009.03.023
Bano, S.; Javed, K.; Ahmad, S.; Rathish, I. G.; Singh, S.; Chaitanya, M.; Arunasree, K. M.; Alam, M. S. Eur J Med Chem 2013, 65, 51–59. DOI: https://doi.org/10.1016/j.ejmech.2013.04.056
Plazoni?, A.; Bucar, F.; ?eljan, M.; Mornar, A.; Nigovi?, B.; Kujund?i?, N. Molecules 2009, 14, 2466–2490. DOI: https://doi.org/10.3390/molecules14072466
Singh, M.; Kaur, M.; Silakari, O. Eur J Med Chem 2014, 84, 206–239. DOI: https://doi.org/10.1016/j.ejmech.2014.07.013
Andersen, O. M.; Markham, K. R.; Flavonoids: Chemistry, Biochemistry and Applications. Taylor and Francis. Boca Raton, EUA, 2006. DOI: https://doi.org/10.1201/9781420039443
Monroy, C.; Castillo, P. Plantas medicinales utilizadas en el estado de Morelos. 2a edición. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Universidad Autónoma del Estado de Morelos. Cuernavaca, Mor, 2007.
Linares, E.; Bye, R. Biodiversitas 2008, 8–11.
Hernández-Silva, D. A.; Cortés-Díaz, E.; Zaragoza-Ramírez, J. L.; Martínez-Hernández, P. A.; González-Bonilla, G. T.; Rodríguez-Castañeda, B.; Hernández-Sedas, D. A. Acta Zool Mex 2011, 27, 47–66. DOI: https://doi.org/10.21829/azm.2011.271733
Guízar, N. E.; Sánchez, V. A. Guía para el reconocimiento de los principales arboles del Alto Balsas. 1991.
Aldana, L.; Salinas, D. O.; Valdés, M. E.; Gutiérrez, M.G. V. Polibotánica 2010, 29, 149–158.
Lautié, E.; Quintero, R.; Fliniaux, M. A.; Villarreal, M. L. J Ethnopharmacol 2008, 120, 402–412. DOI: https://doi.org/10.1016/j.jep.2008.09.014
Cárdenas, R.; Serrano, J. J. R.; Llanos-Romero, E.; Aguirre-Hernández, E.; Herrera-Santoyo, J.; Zúñiga, B.; Rodarte, B.; Alba-Lois, L.; Guevara-Fefer, P. J Entomol 2012, 9, 115–122.
Romero-Estrada, A.; Maldonado-Magaña, A.; González-Christen, J.; Marquina, S.; Garduño-Ramírez, M. L.; Rodríguez-López, V.; Alvarez, L. BMC Complement Altern Med 2016, 16, 1–10. DOI: https://doi.org/10.1186/s12906-016-1397-1
Columba-Palomares, M. C.; Villareal, M. L.; Acevedo Quiroz, M. E.; Marquina Bahena, S.; Alvarez, L. P.; Rodríguez-López, V. Pharmacogn Mag 2015, 11, 322–328. DOI: https://doi.org/10.4103/0973-1296.166067
Parra-Delgado, H.; Ruiz Garcia, G.; Nieto Camacho, A.; Martínez-Vazquez, M. Rev Soc Quím Méx 2004, 48, 293–295.
Pezzuto and Suffness. Assays related to cancer drug discovery. Academic Press. London, 1991.
Chibli, L. A.; Rodrigues, K. C.; Gasparetto, C. M.; Pinto, N. C.; Fabri, R. L.; Scio, E.; Alves, M. S.; Del-Vechio-Vieira, G.; Sousa, O. V. J Ethnopharmacol 2014, 154, 330–338. DOI: https://doi.org/10.1016/j.jep.2014.03.035
Blain, E. J.; Ali, A. Y.; Duance, C. V. Phytother Res 2010, 24, 905–912. DOI: https://doi.org/10.1002/ptr.3055
Navpreet, K.; Chaudhary, J.; Akash, J.; Lalit, K. Int J Pharm Sci Res 2011, 2, 2259–2265.
Nakanishi, T.; Inatomi, Y.; Arai, S.; Yamada, T.; Fukatsu, H.; Murata, H.; Inada, A.; Matsuura, N.; Ubukata, M.; Murata, J.; Iinuma, M.; Perez Farrea, M. A.; Tanaka, T. Heterocycles 2003, 60, 2077–2083. DOI: https://doi.org/10.3987/COM-03-9822
McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, Fitzgerald GA. Proc Natl Acad Sci USA 1999, 96, 272–277. DOI: https://doi.org/10.1073/pnas.96.1.272
Martel-Pelletier, J.; Lajeunesse, D.; Reboul, P.; Pelletier, J. P. Ann Rheum Dis 2003, 62, 501–509. DOI: https://doi.org/10.1136/ard.62.6.501
Shashank, K.; Abhay, K. Sci World J 2013, 4, 32–48. DOI: https://doi.org/10.1016/S0262-1762(13)70190-1
Zhou, Z.; Wei, X.; Fu, H.; Luo, Y. Fitoterapia 2013, 88, 91–95. DOI: https://doi.org/10.1016/j.fitote.2013.05.007
Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J. T.; Bokesch, H.; Kenney, S.; Boyd, M. R. ?J Natl Cancer Inst 1990, 82, 1107–1112. DOI: https://doi.org/10.1093/jnci/82.13.1107
Kuete, V.; Dzotam, J. K.; Vounkeng, I. K.; Fankam, A. G.; Efferth, T. BMC Complement Altern Med 2016, 5, 1–12. DOI: https://doi.org/10.1186/s40064-016-3361-4

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









