Identification, synthesis and structure assignment of two impurities of Erlotinib, a drug used for the treatment of some types of cancer.
DOI:
https://doi.org/10.29356/jmcs.v63i1.714Keywords:
Eritronib, Cancer, Isomers, ImpurityAbstract
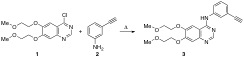
In the synthesis and industrial production of Erlotinib, the formation of organic impurities has been detected, so the identification and control of these is a critical point within the process. Standards for the consumption of pharmaceutical products are well established for active substances. In this sense, the synthesis and unequivocal identification of two impurities of Erlotinib as structural isomers was developed in the present study. The structural characterization of both isomers was confirmed by 1H and 13C-NMR as well as by their X-ray diffraction study.
Downloads
References
Harris, C.; Hennequin, L.; Willerval, O. Tetrahedron Lett.2009, 50, 1600-1602. DOI: https://doi.org/10.1016/j.tetlet.2009.01.099
Chandregowda, V.; Rao, V. G.; Reddy, G. C. Org. Process Res. Dev. 2007, 11, 813-816. DOI: https://doi.org/10.1021/op700054p
Zhang, G.; Zha, L. Res. Chem. Intermed. 2013, 39, 2303–2309. DOI: https://doi.org/10.1007/s11164-012-0757-9
Bridjes, A. J. Chem. Rev. 2001, 101, 2541-2572. DOI: https://doi.org/10.1021/cr000250y
Bagul, C.; Rao, G. K.; Makani, V. K. K.; Tamboli, J. R.; Pal-Bhadra, M.; Kamal, A. Med. Chem. Commun. 2017, 8, 1810-1816. DOI: https://doi.org/10.1039/C7MD00193B
Schnur, R. C.; Arnold, L. D. from PCT Int. Appl. (1996), WO 9639347 A1 Oct 03, 1996.
Q3A(R2) Impurities in New Drug Substances, ICH Tripartite Guideline.
Volk, B.; Dancsó, A.; Bakó, T.; Peltz, C.; Simig, G. Org. Process Res. Dev. 2011, 15, 339-342. DOI: https://doi.org/10.1021/op100241h
Crystallographic data was deposited at Cambridge Crystallographic Data Center (CCDC 1535571 for 4 and 1535572 for 5).
Crossland, R. K.; Servis, K. L. J. Org. Chem. 1970, 35, 3195-3196. DOI: https://doi.org/10.1021/jo00834a087

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









