GC/MS analysis and bioactive properties of extracts obtained from Clusia minor L. leaves
DOI:
https://doi.org/10.29356/jmcs.v62i4.544Keywords:
Clusia minor L., sterols, triterpenoids, antioxidant, anti-inflammatory, cytotoxicityAbstract
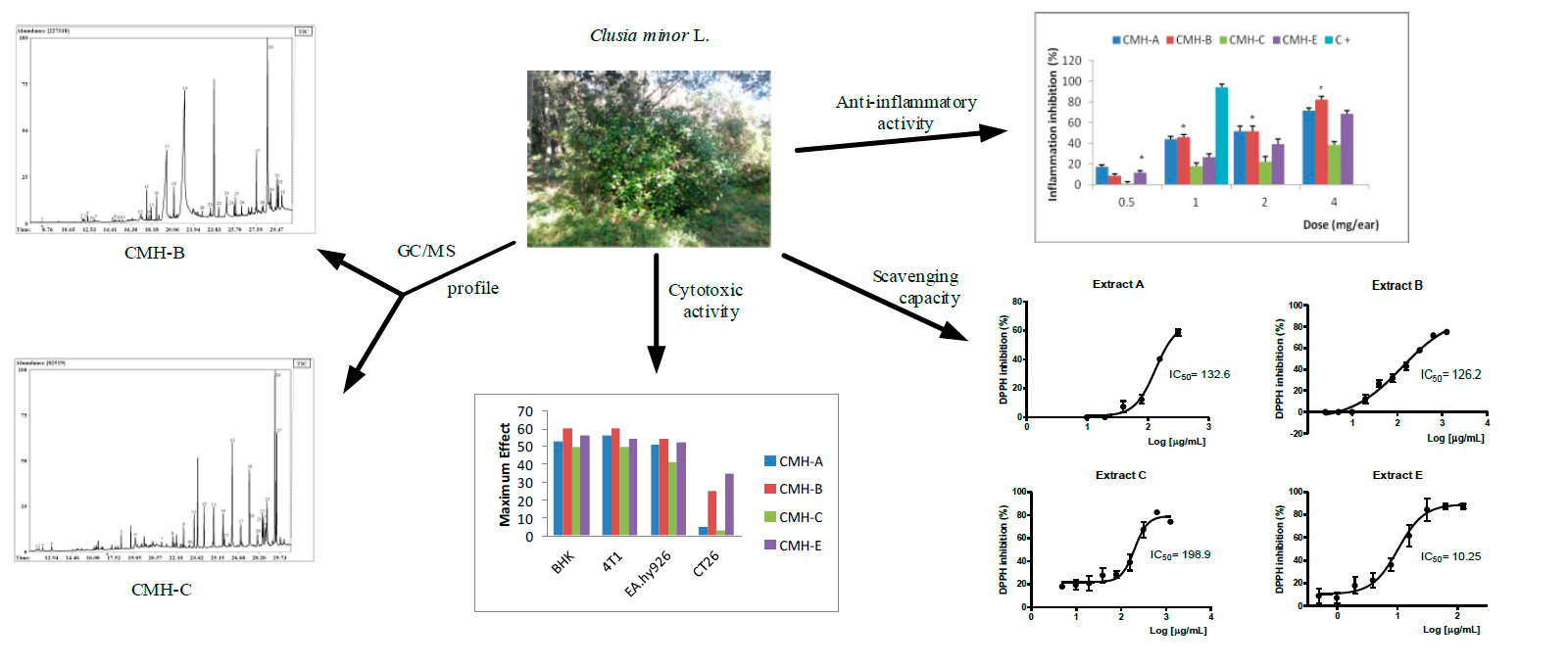
Clusia minor L. is traditionally used to treat many disorders that including pain and inflammation such as sores and warts. Four extracts from the leaves of plant were prepared: hexane (CMH-A), ethyl acetate (CMH-B), methanol (CMH-C) and ethanol (CMH-E) and the pharmacological (antioxidant and anti-inflammatory properties) and toxicity effects were examined. Previously, the main constituents from CMH-A extract was revealed. Here, we present the GC/MS analysis of CMH-B and CMH-C. Thirty three compounds were identified in the CMH-B extract and twenty seven compounds in the CMH-C. The presence of D-α-tocopherol and lupeol was relevant in both extracts. The only sterols identified were sitosterol and stigmasterol. All of them showed effective radical scavenger properties in the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay, being CMH-E extract the most promissory (IC50 = 10.25 µg/mL). CMH-A, C and E extracts, administered topically (0.5–4 mg per ear), significant reduced ear edema induced by croton oil at 4 mg per ear, meanwhile CMH-B that was be able to significant reduce the inflammation at the dose of 2 mg per ear. We evaluated also the cytotoxic activity of the extracts against kidney cells (BHK), colon cancer (CT26), endothelial cancer cells (EA.hy926) and breast cancer (4T1). CMH-B extract showed the most cytotoxicity effect, with IC50 values in the range of 32.01-203.5 µg/mL. In addition, no oral acute toxicity after mice exposure to Clusia minor L. extracts was observed. The results suggest Clusia minor L. may be a good potential source of new bioactive agents for developing medicinal agents.
Downloads
References
Nogueira P.C.; Bittrich V.; Shepherd G.J.; Lopes A.V.; Marsaioli A.V. Phytochemistry 2001, 56, 443-452. DOI: https://doi.org/10.1016/S0031-9422(00)00213-2
Peraza-Sánchez S.R.; Pacheco F.; Noh-Chimal A. Fitoterapia 2007, 78, 315-318. DOI: https://doi.org/10.1016/j.fitote.2007.03.013
Lastres M.; Ruiz-Zapata T.; Castro M.; Torrecilla P.; Lapp M.; Hernández-Chong L.; Muñoz D. Pittieria. 2015, 39, 59-89.
Compagnone R.S.; Suarez A.C.; Leitão S.G.; Delle Monache F. Engl. Braz. J. Pharmacog. 2008, 18, 6-10. DOI: https://doi.org/10.1590/S0102-695X2008000100003
Cuesta-Rubio O.; Piccinelli A.L.; Rastrelli L. Stud. Nat. Prod. Chem. 2005, 32, 671–720. DOI: https://doi.org/10.1016/S1572-5995(05)80066-3
Popolo A.L.; Piccinelli S.M.; Rosalinda S.; Cuesta Rubio O.; Luca R.; Pinto A. Can. J. Physiol. Pharmacol. 2011, 89, 50-57. DOI: https://doi.org/10.1139/Y10-100
Teixeira J.S.R.; Cruz F.G. Tetrahedron Lett. 2005, 46, 2813–2816. DOI: https://doi.org/10.1016/j.tetlet.2005.02.121
Bailón-Moscoso N.; Romero-Benavides J.C.; Sordo M.; Villacís J.; Silva R.; Celi L.; Martínez-Vázquez M.; Ostrosky-Wegmanaet P. Braz. J. Pharmacog. 2016, 26, 44-49. DOI: https://doi.org/10.1016/j.bjp.2015.08.014
Ferreira R. O.; Carvalho Junior A. R.; Riger C.J.; Castro R.N.; Silva T. M. S.; Carvalho M. G. Quim. Nova 39, 2016, 1093-1097.
Ribeiro P.R.; Ferraz C.G.; Guedes M.L.S.; Martins D.; Cruz F.G. Fitoterapia 2011, 82, 1237–1240. DOI: https://doi.org/10.1016/j.fitote.2011.08.012
Nunes J.S.F.; da Silva J.P.; Conserva L.M.; Barreto E. Chin. J. Nat. Med. 2013, 11, 385?390. DOI: https://doi.org/10.1016/S1875-5364(13)60056-4
Nagem T.J.; Mesquita A.A.L.; Silva R. Fitoterapia 1993, 64, 380.
Henry G.E.; Jacobs H.; Carrington C.M.S.; McLean S.; Reynolds W. Tetrahedron Lett. 1999, 55, 1581-1596. DOI: https://doi.org/10.1016/S0040-4020(98)01203-4
Ito C.; Itoigawa M.; Miyamoto Y.; Onoda S.; Rao K.; Mukainaka T.; Tozuda H.; Nishino H.; Furukawa H. J. Nat. Prod. 2003, 66, 206-209. DOI: https://doi.org/10.1021/np020372g
Mangas R.; Bello A.; Cuesta-Rubio O.; Piccinelli A.L.; Rastrelli L. Lat. Am. J. Pharm. 2008, 27, 762-775.
Mangas R.; Montes de Oca R.; Bello A.; Vázquez A.N. Lat. Am. J. Pharm. 2008, 27, 747-751.
Tabart J. Food Chem. 2009, 133, 1226-1233. DOI: https://doi.org/10.1016/j.foodchem.2008.08.013
Mosmann T. J. Immunol. Methods 1983, 65, 55-63. DOI: https://doi.org/10.1016/0022-1759(83)90303-4
Griswold D.E.; Martin L.D.; Badger A.M.; Breton J.; Chabot-Fletcher M. Inflamm. Res. 1998, 47, 56-61. DOI: https://doi.org/10.1007/s000110050270
OECD Guideline for the testing of chemicals, No. 423. Acute oral toxicity acute toxic class method. Paris, France: Organization for Economic Cooperation and Development. 2000.
Neerman M.F. Inter. J. Aromather. 2003, 13, 114-120. DOI: https://doi.org/10.1016/S0962-4562(03)00078-X
Mathur S.B. Phytochemistry. 1972, 11, 1513-1514. DOI: https://doi.org/10.1016/S0031-9422(00)90127-4
Hasbun-Pacheco C.; Calvo-Pineda M.A.; Barrios-Chica M.; Arguedas-Campos E.; Calvo A.; Jiménez R.; Poveda-Alvarez L.J. Ing. Cienc. Química. 1985, 9, 96-97.
Farfan M.; Martinez E.; Delle Monache F. Rev. Colomb. Quim 1998, 27, 87-89.
Bouic, P.J. Curr. Opin. Clin. Nutr. Metab. Care. 2001, 4, 471-475.
Bouic P.J.; Lamprecht J.H. Altern. Med. Rev. 1999, 4, 170-177.
Loizou S.; Lekakis I.; Chrousos G.P.; Moutsatsou P. Mol. Nutr. Food Res. 2010, 54, 551-558. DOI: https://doi.org/10.1002/mnfr.200900012
Cao G.; Sofic, E.; Prior R.L. Free Radic. Biol. Med. 1997, 22, 749-760. DOI: https://doi.org/10.1016/S0891-5849(96)00351-6
Chem Z.Y.; Cham P.T.; Ho, K.Y.; Fung K.P.; Wang J. Chem. Phys. Lipids. 1996, 79, 157-163. DOI: https://doi.org/10.1016/0009-3084(96)02523-6
Heim K.; Tagliaferro A.; Bobilya D.J. J. Nutr. Biochem. 2002, 13, 572-584. DOI: https://doi.org/10.1016/S0955-2863(02)00208-5
Se-Youm A.; Asres K.; El-Fiky F. Phytochemistry 2006, 67, 2058-2070. DOI: https://doi.org/10.1016/j.phytochem.2006.07.002
Van A.S.; Van D.; Tromp M.; Griffioen D.; Van B.; Van D.V.; Bast A. Free Radic. Biol. Med. 1996, 20, 331-342. DOI: https://doi.org/10.1016/0891-5849(95)02047-0
Singh K.; Bhori M.; Marar T. Hum. Exp. Toxicol. 2015, 34, 380-389. DOI: https://doi.org/10.1177/0960327114533577
Hamdan D.; El-Readi M.Z.; Tahrani A.; Herrmann F.; Kaufmann D.; Farrag N.; El-Shazly A.; Wink M. Z. Naturforsch C. 2011, 66, 385. DOI: https://doi.org/10.5560/ZNC.2011.66c0385
Kaur N.; Chaudhary J.; Jain A.; Kishore L. Int. J. Pharm. Sci. Res. 2011, 2, 2259-2265.
Baskar A.A.; Al Numair K.S.; Paulraj M.G.; Alsaif M.A.; Al Muamar M.; Ignacimuthu, S.; J Med. Food. 2012, 15, 335-343. DOI: https://doi.org/10.1089/jmf.2011.1780
Vivancos M.; Moreno J.J. Free Radic. Biol. Med. 2005, 39, 91-97. DOI: https://doi.org/10.1016/j.freeradbiomed.2005.02.025
Gupta A.; Sharma A.K.; Dobhal M.P.; Sharma M.C.; Gupta R.S J Diabetes. 2011, 3, 29-37. DOI: https://doi.org/10.1111/j.1753-0407.2010.00107.x
Raman B.V.; La S.; Saradhi M.P.; Rao B.N.; Khrisna A.N.V.; Sudhakar M.; Radhakrishnan T. Asian J. Pharm. Clin. Res. 2012, 5, 99-106.
Kuete V.; Krusche B.; Youns M.; Voukeng I.; Fankam A.G.; Tankeo S.; Lacmata S.; Effert T. J. Ethnopharmacol. 2011, 134, 803–812. DOI: https://doi.org/10.1016/j.jep.2011.01.035
Gallo M.B.C.; Sarachine M.J. Int. J. Biomed. Pharmaceut. Sci. 2009, 3, 46-66.
Hernández L.; Palazon J.; Navarro-Ocaña A. in: Phytochemicals-A Global Perspective of Their Role in Nutrition and Health. Dr. Venketeshwer, R. Ed., InTech, Rijeka, Croatia, 2012, 487-510.
Ju Y.H.; Clausen L.M.; Allred K.F.; Almada A.L.; Helferich W.G. J. Nutr. 2004, 134, 1145-1151. DOI: https://doi.org/10.1093/jn/134.5.1145
Ovesna Z.; Vachalkova A.; Horvathova K. Neoplasma 2004, 51, 407-414. DOI: https://doi.org/10.1177/0264550504048337

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









