Effect of Polymers nature and Stirring Speeds on Physicochemical Properties and the Controlled Release of Allopurinol-loaded Microspheres
DOI:
https://doi.org/10.29356/jmcs.v66i1.1583Keywords:
Allopurinol, microencapsulation, carrier polymer, stirring rates, controlled releaseAbstract
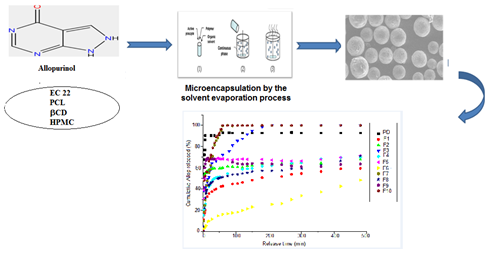
Abstract. Allopurinol is an antigout drug therapy, commonly used in the treatment of chronic gout or hyperuricaemia associated with treatment of diuretic conditions. In the present study, new formulations based on Allopurinol, have been prepared with the microencapsulation by solvent evaporation process. Microspheres were prepared using pure Allopurinol and polymeric matrices (ethylcellulose EC, poly (ε-caprolactone) PCL, β-cyclodextrin CD and hydroxypropylmethylcellulose HPMC) at different compositions and stirring speeds to investigate the effect of these parameters on loading efficiency and drug release kinetics. The formulations produced were characterized by various methods : Fourier transforms infrared spectroscopy (FTIR), X-ray powder diffractometry, optical microscopy, surface morphology by scanning electron microscopy (SEM) and drug loading, as well as in vitro release studies in the simulated stomach tract. Depending on the stirring speed and the composition of the microparticles, the active ingredient loading is in a range from 10.46 ± 1.45 to 46.40 ± 0.5%. The microspheres are spherical and the mean Sauter diameter (d32) of the microparticles obtained is smaller and is in the range of 47.71 to 151.01 µm. Different release profiles were obtained and show that the release rate is strongly influenced by the characteristics of the microparticles ; namely, the stirring rates and the composition of the microparticles. The release mechanism was identified by modelling using Higuchi and Korsmeyer-Peppas models.
Resumen. Alopurinol es una droga terapéutica para tratar la gota, y se utiliza en el tratamiento de gota crónica o hiperuricemia asociada con el tratamiento de condiciones diuréticas. En este estudio, nuevas formulaciones basadas en Alopurinol se prepararon mediante microencapsulación por el proceso de evaporación de disolvente. Microesferas se prepararon usando Alopurinol puro y diferentes matrices poliméricas (etil-celulosa EC, poli(-caprolactona) PCL, β-cyclodextrina CD e hidroxipropil-metil-celulose HPMC) en diferentes composiciones y velocidades de agitación, para investigar el efecto de estos parámetros en la eficiencia de carga y en la cinética de liberación del fármaco. Las formulaciones obtenidas fueron caracterizadas por diferentes técnicas : Espectroscopía infrarroja de transformadas de Fourier (FTIR), difractometría de rayos X de polvos, microscopía óptica, morfología de superficies mediante microscopía electrónica de barrido electrónico, y la eficiencia de carga del fármaco, así como estudios de liberación in vitro en tracto estomacal simulado. Dependiendo de la velocidad de agitación y la composición de las micropartículas, la carga del ingrediente activo se encuentra en el rango de 10.46 ± 1.45 a 46.40 ± 0.5%. Las microesferas son esféricas y el diámetro medio de Sauter (d32) de las micropartículas obtenidas es menor, y se encuentra en el rango de 47.71 a 151.01 µm. Se obtuvieron diferentes perfiles de liberación y se observa que la velocidad de liberación está influenciada principalmente por las características propias de la producción de las micropartículas ; en particualr, las velocidades de agitación y las composición de las micropartículas. El mecanismo de liberación se ajusta mejor a los modelos matemáticos de Higuchi and Korsmeyer-Peppas.
Downloads
References
Pacher, P.; Nivorozhkin, A.; Szabó, C. Pharmacol. Rev. 2006, 58, 87–114. DOI: https://doi.org/10.1124/pr.58.1.6
Vazquez-Mellado, J.; Morales, E. M.; Pacheco-Tena, C.; Burgos-Vargas, R. Ann. Rheum. Dis. 2001, 60, 981–983. DOI: https://doi.org/10.1136/ard.60.10.981
Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari, R.; Deb, P. K.; Kalia, K.; et al. Int.l J. Pharm. 2018, 548, 540–558. DOI: https://doi.org/10.1016/j.ijpharm.2018.07.027
Sy, P. M.; Anton, N.; Idoux-Gillet, Y.; Dieng, S. M.; Messaddeq, N.; Ennahar, S.; et al. Int. J. Pharm.. 2018, 549, 299–305. DOI: https://doi.org/10.1016/j.ijpharm.2018.07.066
Hu, Y.; Zhi, Z.; Wang, T.; Jiang, T.; Wang, S. Eur. J. Pharm. Biopharm. 2011, 79, 544–551. DOI: https://doi.org/10.1016/j.ejpb.2011.07.001
Lu, M.; Cao, Y.; Ho, C.-T. & Huang, Q. J. Agric. Food Chem. 2016, 64, 4735–4741. DOI: https://doi.org/10.1021/acs.jafc.6b01095
Khan, S. A.; Ahmad, M.; Murtaza, G.; Aamir, M. N.; Madni, A.; Kousar, R.; Minhas, U. Ars Pharm. 2010, 51, 105-115.
Sharma, O. P.; Shah, M. V.; Parikh, D. C.; Mehta, T. A. Expert Opin. Drug Delivery. 2015, 12, 513–524. DOI: https://doi.org/10.1517/17425247.2014.944861
El-Gibaly, I.; Abdel-Ghaffar, S. Int. J. Pharm. 2005, 294, 33–51. DOI: https://doi.org/10.1016/j.ijpharm.2004.12.027
Jagdale, S. C.; Musale, V.; Kuchekar, B. S.; Chabukswar, A. R. Braz. J. Pharm. Sci. 2011, 47, 513-523. DOI: https://doi.org/10.1590/S1984-82502011000400028
Rowe, R. C.; Sheskey, P. J.; Quinn, M. E. Handbook of Pharmaceutical Excipients. Edit. London: Pharmaceutical Press; 2009.
Giri, T. K.; Kumar, K.; Alexander, A.; et al. Bull Fac. Pharm. Cairo Univ. 2012, 147–159. DOI: https://doi.org/10.1016/j.bfopcu.2012.07.002
Diaf, K.; Elbahri, Z.; Chafi, N.; Belarbi, L.; Mesli, A. Chem. Pap. 2012, 66:779. DOI: https://doi.org/10.2478/s11696-012-0191-x
Vueba, M. L.; Batista de Carvalho, L. A. E.; Veiga, F.; Sousa, J. J.; Pina, M. E. Eur. J. Pharm. Biopharm. 2004, 58, 51-59. DOI: https://doi.org/10.1016/j.ejpb.2004.03.006
Poovi, G.; Rajpriyadarsini, S.; Uma, S.; Vinothini, R. Asian J. Pharm. Sci. 2015, 10, 433-441. DOI: https://doi.org/10.1016/j.ajps.2015.05.001
Merdoud, A.; Mouffok, M.; Mesli, A.; Chafi, N.; Chaib, M. J. Serb. Chem. Soc. 2020, 85, 531–545. DOI: https://doi.org/10.2298/JSC190326132M
Mouffok M.; Mesli A.; Abdelmalek I.; Gontier E. J. Serb. Chem. Soc. 2016, 81,1183. DOI: https://doi.org/10.2298/JSC160308068M
Larbi, O. C.; Merine, H.; Ramli, Y.; Toumi, F. B.; Guemra, K.; Dehbi, A. J. Serb. Chem. Soc. 2018, 83, 1–19. DOI: https://doi.org/10.2298/JSC171112065LA
Bala, S.; Mahatma, O. P.; Azim, Md. S. Inter. Res. J. Pharm. 2013, 4,77. DOI: https://doi.org/10.7897/2230-8407.04916
Khoukhi, O.; El Bahri, Z.; Diaf, K.; Baitiche, M. Chem. Pap. 2016, 0014.
Azouz, L.; Dahmoune, F.; Rezgui, F.; G'Sell, C. Mater. Sci. Eng. 2016, 58,412-419. DOI: https://doi.org/10.1016/j.msec.2015.08.058
Spadola, G.; Sanna, V.; Bartoli, J.; et al. Environ. Sci. Pollut. Res. 2020, 27, 20125–20135. DOI: https://doi.org/10.1007/s11356-020-08532-7
Zhu, K. J.; Li, Y.; Jiang, H. L.; Yasuda, H.; Ichimaru, A.; Yamamoto, K.; Lecomte, P.; Jerome, R. J. Microencapsul. 2005, 22, 25-36. DOI: https://doi.org/10.1080/02652040400026350
Middleton, JC.; Tipton, AJ. Biomaterials.. 2000, 21, 2335-46. DOI: https://doi.org/10.1016/S0142-9612(00)00101-0
Greenwald, D.; Shumway, S.; Albear, P.; Gottlieb, L. J. Surg. Res. 1994, 56, 372 – 7. DOI: https://doi.org/10.1006/jsre.1994.1058
Nair, L. S.; Laurencin, C. T. Prog. Polym. Sci. 2007, 32, 762-98. DOI: https://doi.org/10.1016/j.progpolymsci.2007.05.017
Rudkevich, D. M.; Leontiev, A. V. Aust. J. Chem. 2004, 57, 713-722. DOI: https://doi.org/10.1071/CH04102
Mourtzinos, I.; Fotini, S.; Yannakopoulou, K.; Chiou, A.; Karathanos, V. T. J. Agric. Food Chem. 2007, 55, 8088. DOI: https://doi.org/10.1021/jf0709698
Nalluri, B. N.; Chowdary, K. P. R.; Murthy, K. V. R.; Becket, G.; Crooks, P. A. AAPS Pharm. Sci. Tech. 2007, 8, E1-E7. DOI: https://doi.org/10.1208/pt0802036
Samy, A.; Marzouk, M. A.; Ammar, A. A.; Ahmed, M. K. Drug Discoveries Ther. 2010, 4,77-84.
Wagner, J. G. J. Pharm. Sci. 1969, 58, 1253. DOI: https://doi.org/10.1002/jps.2600581021
Gibaldi, M.; Feldman, S. J. Pharm. Sci. 1967, 56, 1238. DOI: https://doi.org/10.1002/jps.2600561005
Higuchi, T. J. Pharm. Sci. 1963, 52, 1145–9. DOI: https://doi.org/10.1002/jps.2600521210
Korsmeyer, R. W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N. A. Inter. J. Pharm. 1983, 15, 25–35. DOI: https://doi.org/10.1016/0378-5173(83)90064-9
Garud, N.; Garud, A. Trop. J. Pharm. Res. 2012, 11, 577-583. DOI: https://doi.org/10.4314/tjpr.v11i4.8
Le Corre, P.; Le Guevello, P.; Gajan, V.; Chevanne, F.; LeVerge, R. Int. J. Pharm. 1994, 107, 41-49. DOI: https://doi.org/10.1016/0378-5173(94)90300-X
Dhanaraju, M. D.; Elizabeth, S.; Poovi, G. J. Pharm. Investig. 2011, 41, 279–288. DOI: https://doi.org/10.4333/KPS.2011.41.5.279
Blanc, M. D., Gomez, C., Olmo, R.; et al. Int. J. Pharm. 2000, 202, 29–39. DOI: https://doi.org/10.1016/S0378-5173(00)00408-7
Szejtli, J. Cyclodextrin Technology, Kluwer Academic Publishers, Dordrecht. 1988, 1-78. DOI: https://doi.org/10.1007/978-94-015-7797-7_1
Stella, V. J., Rao, V. M., Zannou, E. A.; Zia, V. Adv. Drug Deliv. Rev. 1999, 36, 3-16. DOI: https://doi.org/10.1016/S0169-409X(98)00052-0
Bodmeier, R.; McGinity, J.W. Int. J. Pharm. 1988, 43, 179-186. DOI: https://doi.org/10.1016/0378-5173(88)90073-7
Bodmeier, R.; Chen, H. J. Controlled Release. 1989, 10, 167-175. DOI: https://doi.org/10.1016/0168-3659(89)90059-X
Jagtap, Y. M.; Bhujbal, R. K.; Ranade, A. N.; et al. Indian J. Pharm. Sci. 2012, 74, 512–520. DOI: https://doi.org/10.4103/0250-474X.110578
Silva Pires, M. A.; Souza dos Santos, R. A.;Sinisterra, R. D. Molecules. 2011, 16, 4482. DOI: https://doi.org/10.3390/molecules16064482
Okonogi, S.; Oguchi, T.; Yonemochi, S.; Puttipipatkhacharm, S.; Yamamoto, K. Int. J. Pharm. 1997, 156, 175-180. DOI: https://doi.org/10.1016/S0378-5173(97)00196-8
Zhang,G. G. Z.; Law, D.; Schmitt, E. A.; Qiu,Y. Adv. Drug Deliv.Rev. 2004, 56, 371-390. DOI: https://doi.org/10.1016/j.addr.2003.10.009
Sun, Y.;Rui, Y.; Wenliang, Z.; Tang, X. Int. J. Pharm. 2008, 359, 144-149. DOI: https://doi.org/10.1016/j.ijpharm.2008.03.040
Mura, P.; Manderioli, A.; Bramanti, G.; Ceccarelli, L. Drug Dev. Ind. Pharm. 1996, 22, 909–916. DOI: https://doi.org/10.3109/03639049609065920
Jeong, J. C.; Lee, J.; Cho, K. J. Control. Rel. 2003, 92, 249-258. DOI: https://doi.org/10.1016/S0168-3659(03)00367-5
Yunpeng, C.; Yinghui, C.; Xiaoyun, H.; Zhenguo, L.; Weien, Y. Int. J. Nanomedicine. 2013, 8, 1111-1120.
Atkins, P.W. Physical Chemistry, sixthed., Oxford University Press, Oxford,UK. 1998.
Gautier, H.;Guicheux, J.; Grimandi, G.; Faivre, A.; Daculsi, G.; Merle, C. J. Biomed. Mater.Res. 1998, 40, 606 -613. DOI: https://doi.org/10.1002/(SICI)1097-4636(19980615)40:4<606::AID-JBM12>3.0.CO;2-D
Mateovic, T.; Kriznar, B.; Bogataj, M.; Mrhar, A. J. Microencapsul. 2002, 19, 29-36. DOI: https://doi.org/10.1080/02652040010055289
Yang, Y.Y.; Chung, T.S.; Ng, N.P. Biomaterials. 2001, 22, 231-241. DOI: https://doi.org/10.1016/S0142-9612(00)00178-2
Mura, P.; Faucci, M.T.; Parrini, P.L. Drug Dev. Ind. Pharm. 2001, 27, 119-128. DOI: https://doi.org/10.1081/DDC-100000478
Samy, E. M.; Hassan, M. A.; Tous, S. S.; Rhodes, C. T. Eur. J. Pharm. Biopharm. 2000, 49, 119-127. DOI: https://doi.org/10.1016/S0939-6411(99)00079-X
Lloyd, G. R.; Craig, D. Q. M.; Smith, A. A. Eur. J. Pharm. Biopharm. 1999, 48, 59-65. DOI: https://doi.org/10.1016/S0939-6411(99)00022-3
Pokharkar, V. B.; Mandpe, L. P.; Padamwar, M. N.; et al. Powder Technol. 2006, 167, 20-25. DOI: https://doi.org/10.1016/j.powtec.2006.05.012
Siepmann, J.; Peppas, N. A. Adv. Drug Deliv. Rev. 2001, 48, 139-157. DOI: https://doi.org/10.1016/S0169-409X(01)00112-0
Ritger, P. L.; Peppas, N. A. J. Control. Rel. 1987, 5, 37-42. DOI: https://doi.org/10.1016/0168-3659(87)90035-6
Soppimath, K. S.; Kulkarni, A. R.; Aminabhavi, T. M. J. Microencapsul. 2001, 18, 397-409. DOI: https://doi.org/10.1080/02652040010018083

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









