GC-MS Analysis and Biological Activities of Algerian Salvia microphylla Essential Oils
DOI:
https://doi.org/10.29356/jmcs.v65i4.1581Keywords:
Salvia microphylla, GC-MS analysis, antioxidant, anticholinesterase, antimicrobialAbstract
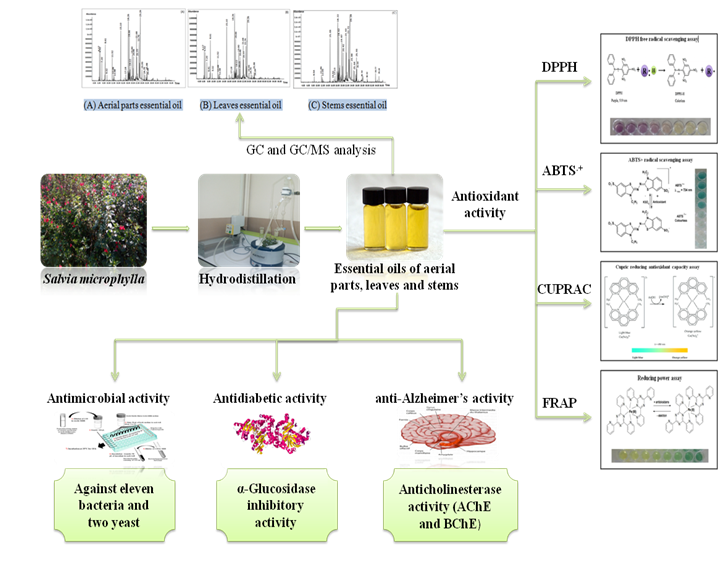
Abstract. Salvia microphylla is a known species due to its broad uses in traditional medicine against memory loss and rheumatism. The knowledge regarding the chemical composition and biological activities of the species collected in Algeria, no studies have been reported in the literature. Therefore, the present work focuses on the characterization of the chemical composition of the essential oils (EOs) and the determination of the antioxidant, anticholinesterase, α-glucosidase, and antimicrobial activities of Salvia microphylla. The EOs were obtained by hydrodistillation from the aerial parts, leaves and stems and submitted to chemical analysis by GC and GC/MS. The β-Caryophyllene was identified as the main constituent in the aerial parts and leaves essential oils with 16.75 ± 0.02 % and 17.86 ± 0.07 %, respectively. Likewise, the α-Eudesmol was the predominant component in the stems oil with (21.47 ± 0.20 %). The antioxidant activity of EOs was estimated through using four comparative methods: DPPH, ABTS•+, Reducing power and CUPRAC assays. The Stems oil was the most active one in CUPRAC assay, with an IC50 value with 7.72 ± 0.43 µg/mL. The enzyme inhibitory activity of the essential oils was realized against key enzymes involved in type 2 diabetes (α-glucosidase) using 4-Nitrophenyl-α-d-glucopyranoside as substrate and in neurodegenerative (AChE and BChE) diseases. The highest anticholinesterase activity against acetylcholinesterase was observed in the EO of aerial parts essential (IC50: 23.65 ± 0.73 µg/mL). The EO isolated from stems (IC50: 37.07 ± 1.44 µg/mL) exhibited a butyrylcholinesterase activity very close to that of analytical standard galantamine (IC50: 34.75 ± 1.99 µg/mL). Furthermore, all EOs displayed high inhibitory activity against α-glucosidase, better to that of the standard acarbose. The EOs of Salvia microphylla display potential properties against type 2 diabetes. A broth microdilution method was used to evaluate the antimicrobial activity of Salvia microphylla EOs, against eleven microbial strains and two yeast. The EOs showed better antibacterial activity against Gram-positive and Gram-negative bacteria except for Pseudomonas aeruginosa, with the stems essential oil being more efficient. Moreover, significant antifungal activity was observed against Candida albicans.
Resumen. Salvia microphylla es una specie conocida debido a su amplio uso en medicina tradicional, contra la pérdida de memoria y el reumatismo. En el caso de la especie de planta recolectada en Algeria, no hay datos publicados sobre su composición química y sus actividades biológicas. Por ello, el presente trabajo ha sido enfocado en la caracterización de la composición química de aceites esenciales (EOs) de Salvia microphylla y en la determinación de sus actividades antioxidante, anticolinesterasa, α-glucosidasa y antimicrobiano. Los EOs fueron obtenidos mediante hidrodestilación de las partes aéreas, ojas y tallos, y fueron sometidos al análisis por cromatografía de gases con detección por ionización en flama y por espectrometría de masas. Se identificó a β-cariofileno como el componente principal de los aceites de las partes aéreas y de tallos con concentraciones de 16.75 ± 0.02 % y 17.86 ± 0.07 %, respectivamente. Por su parte, el α-Eudesmol fue encontrado como componente predominante en aceite de tallos (21.47 ± 0.20 %). La actividad antioxidante de los EOs fue estimada en base a cuatro métodos compartivos: DPPH, ABTS•+, poder reductor y ensayo CUPRAC. El aceite de tallos resultó ser el más activo en ensayo CUPRAC, con el valor IC50 de 7.72 ± 0.43 µg/mL. La actividad inhibitora de enzimas de los EOs fue evaluada contra principales enzimas involucrados en diabetes tipo 2 (α-glucosidasa), utilizando 4-Nitrofenil-α-d-glucopiranosida como sustrato, y en enfermedades neurodergenerativas (AChE y BChE). La mayor actividad anticolinesterasa y acetilcolinesterasa fue observada en el EO de partes aéreas (IC50: 23.65 ± 0.73 µg/mL). El EO islado de tallos (IC50: 37.07 ± 1.44 µg/mL) presentó actividad de butirilcolinestarasa muy similar a la del estándar analítico, galantamina (IC50: 34.75 ± 1.99 µg/mL). Aunado a ello, todos EOs presentaron una alta actividad inhibitora contra α-glucosidasa, que era mejor comparando con la del estándar de acarbosa. Los EOs de Salvia microphylla presentan potenciales propiedades contra diabetes tipo 2. Para evaluar la actividad antimicrobiana de los EOs de Salvia microphylla, se utilizó el método de microdulución en caldo, contra once sepas microbianas y dos de levadura. La mejor actividad se observó contra bacterias Gram-positivas y Gram-negativas, excepto Pseudomonas aeruginosa, los cuales presentaron alta resistencia. Los EOs presentaron también importante actividad antifungica contra Candida albicans.
Downloads
References
Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar M. Food Chem. Toxicol. 2008, 46, 446-475. DOI: https://doi.org/10.1016/j.fct.2007.09.106
Hedge, I.C. Advances in Labiatae science. 1992, 7-17.
Kabouche, A.; Kabouche, Z. Stud. Nat. Prod. Chem. 2008, 753-833. DOI: https://doi.org/10.1016/S1572-5995(08)80017-8
Medjahed, F.; Merouane, A.; Saadi, A.; Bader, A.; Cioni, P.L.; Flamini, G. Chilean J. Agric. Res. 2016, 76, 195-200. DOI : http://dx.doi.org/10.4067/S0718-58392016000200009 DOI: https://doi.org/10.4067/S0718-58392016000200009
Beladjila, K.A.; Berrehal, D.; Al-Aboudi, A.; Semra, Z.; Al-Jaber, H.; Bachari, K.; Kabouche, Z. Chem. Nat. Compd. 2018, 54, 581-583. DOI : https://doi.org/10.1007/s10600-018-2414-z
?enol, F.S.; Orhan, I.; Celep, F.; Kahraman, A.; Do?an, M.; Yilmaz, G.; ?ener, B. Food Chem. 2010, 120, 34-43. DOI: https://doi.org/10.1016/j.foodchem.2009.09.066
Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. J. Ethnopharmacol. 2008a, 119, 664-672. DOI: https://doi.org/10.1016/j.jep.2008.06.030
Kamatou, G.P.; Van Zyl, R.; Davids, H.; Van Heerden, F.; Lourens, A.; Viljoen, A.M. S. Afr. J. Bot. 2008b, 74, 238-243. DOI: https://doi.org/10.1016/j.sajb.2007.08.001
Kivrak, ?.; Duru, M.E.; Öztürk, M.; Mercan, N.; Harmandar, M.; Topçu, G. Food chem. 2009, 116, 470-479. DOI: https://doi.org/10.1016/j.foodchem.2009.02.069
Tel, G.; Öztürk, M.; Duru, M.E.; Harmandar, M.; Topçu, G. Food Chem. Toxicol. 2010, 48, 3189-3193. DOI : https://doi.org/10.1016/j.fct.2010.08.020
Al-Jaber, H.I.; Al-Qudah, M.A.; Barhoumi, L.M.; Abaza, I.F.; Afifi, F.U. Nat. Prod. Res. 2012, 26, 1179-1187. DOI: https://doi.org/10.1080/14786419.2010.543901
Coisin, M.; Burzo, I.; Stefan, M.; Rosenhech, E.; Zamfirache, M.M. An. Stiint. Univ. Al. I. Cuza Iasi, Sect. II a. Biol. veget. 2012, 58, 51-58.
Marchev, A.; Ivanov, I.; Denev, P.; Nikolova, M.; Gochev, V.; Stoyanova, A.; Pavlov, A.; Georgiev, V. Journal of BioScience & Biotechnology. 2015, 4.
Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Ind. Crops Prod. 2016, 83, 492-499. DOI: https://doi.org/10.1016/j.indcrop.2015.12.080
Temel, H.E.; Demirci, B.; Demirci, F.; Celep, F.; Kahraman, A.; Do?an, M.; Hüsnü Can Ba?er, K. J. Essent. Oil Res. 2016, 28, 322-331. DOI :https://doi.org/10.1080/10412905.2016.1159257
El Euch, S.K.; Hassine, D.; Cazaux, S.; Bouzouita, N.; Bouajila, J. S. Afr. J. Bot. 2019, 120, 253-260. DOI: https://doi.org/10.1016/j.sajb.2018.07.010
Süzgeç-Selçuk, S.; Özek, T.; Özek, G.; Yur, S.; Göger, F.; Gürdal, M.B.; Toplan, G.G.; Meriçli, A.H.; Ba?er, K.H.C. Rec. Nat. Prod. 2021, 15, 10-24. DOI: http://doi.org/10.25135/rnp.185.20.03.1579 DOI: https://doi.org/10.25135/rnp.185.20.03.1579
Crespo, M.E.; Jimenez, J.; Navarro, C.; Zarzuelo, A. Planta Med. 1986, 52, 367-369. DOI: 10.1055/s-2007-969187 DOI: https://doi.org/10.1055/s-2007-969187
Perry, N.S.L.; Houghton, P.J.; Sampson, J.; Theobald, A.E.; Hart, S.; Lis-Balchin, M.; Hoult, J.R.S.; Evans, P.; Jenner, P.; Milligan, S.; Perry, E.K. J. Pharm. Pharmacol. 2001, 53, 1347-1356. DOI: https://doi.org/10.1211/0022357011777846
Jirovetz, L.; Buchbauer, G.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Schmidt, E. Nutrition-Vienna. 2006, 30, 152.
Nikoli?, M.; Jovanovi?, K.K.; Markovi?, T.; Markovi?, D.; Gligorijevi?, N.; Radulovi?, S.; Sokovi?, M. Ind. Crops Prod. 2014, 61, 225-232. DOI: https://doi.org/10.1016/j.indcrop.2014.07.011
Adrar, N.; Oukil, N.; Bedjou, F. Ind. Crops Prod. 2016, 88, 112-119. DOI: https://doi.org/10.1016/j.indcrop.2015.12.007
Bahadori, M.B.; Dinparast, L.; Zengin, G.; Sarikurkcu, C.; Bahadori, S.; Asghari, B.; Movahhedin, N. Int. J. Food Prop. 2017a, 20, 1761-1772. DOI: https://doi.org/10.1080/10942912.2016.1218893
Bahadori, M.B.; Salehi, P.; Sonboli, A. Int. J. Food Prop. 2017b, 20, 2974-2981. DOI: https://doi.org/10.1080/10942912.2016.1263862
Valdés, B.; Martín, M.; Díaz, Z. Bot. Macaron. 1995, 21, 107-120.
Jenks, A.A.; Kim, S.-C. J. Ethnopharmacol. 2013, 146, 214-224. DOI: https://doi.org/10.1016/j.jep.2012.12.035
Béjar, E.; Bussmann, R.; Roa, C.; Sharon, D. Latin Herbal Press, San Diego. 2001.
Lima, R.K.; dasGraças Cardoso, M.; Andrade, M.A.; Guimarães, P.L.; Batista, L.R.; Nelson, D.L. J. Am. Oil Chem. Soc. 2012, 89, 523-528. DOI: https://doi.org/10.1007/s11746-011-1938-1
Romo-Asunción, D.; Ávila-Calderón, M.A.; Ramos-López, M.A.; Barranco-Florido, J.E.; Rodríguez-Navarro, S.; Romero-Gomez, S.; Aldeco-Pérez, E.J.; Pacheco-Aguilar, J.R.; Rico-Rodríguez, M.A. Fla. Entomol. 2016, 345-351. DOI: https://www.jstor.org/stable/24891070 DOI: https://doi.org/10.1653/024.099.0301
Aydo?mu?, Z.; Ye??lyurt, V.; Topcu, G. Nat. Prod. Res. 2006, 20, 775-781. DOI: https://doi.org/10.1080/14786410500462843
Esquivel, B.; Cardenas, J.; Rodriguez-Hahn, L.; Ramamoorthy, T. J. Nat. Prod. 1987, 50, 738-740. DOI: https://doi.org/10.1021/np50052a029
Esquivel, B.; del Socorro Martínez, N.; Cárdenas, J.; Ramamoorthy, T.; Rodríguez-Hahn, L. Planta Med. 1989, 55, 62-63. DOI: 10.1055/s-2006-961827 DOI: https://doi.org/10.1055/s-2006-961827
Bautista, E.; Toscano, R.A.; Ortega, A. Org. Lett. 2013, 15, 3210-3213. DOI: https://doi.org/10.1021/ol401022c
Bautista, E.; Toscano, R.n.A.; Ortega, A. J. Nat. Prod. 2014, 77, 1088-1092. DOI: https://doi.org/10.1021/np4009893
Chialva, F.; Monguzzi, F.; Manitto, P. J. Essent. Oil Res. 1992, 4, 447-455. DOI: https://doi.org/10.1080/10412905.1992.9698108
Koutsaviti, A.; Antonopoulou, V.; Vlassi, A.; Antonatos, S.; Michaelakis, A.; Papachristos, D.P.; Tzakou, O. Journal of pest science. 2018, 91, 873-886. DOI: https://doi.org/10.1007/s10340-017-0934-0
Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Plants. 2020, 9, 691. DOI: https://doi.org/10.3390/plants9060691
National Committee for Clinical Laboratory Standards (NCCLS), Wayne, PA. New York. M2-A6. 1997.
Clinical and laboratory standards institute. Quality Control Minimal Inhibitory Concentration (MIC) Limits for Broth Microdilution and MIC Interpretive Breakpoints. Supplement M27-S2. 2006.
Joulain, D.; König, W. Verlag: EB-Verlag, Hamburg, Germany. 1998.
Adams, R.P. Quadruple mass spectroscopy. Allured Publishing Corporation, Carol. Stream, IL, USA, 2001, 456.
Van Den Dool, H.; Kratz, P. D. A. J. Chromatogr. A. 1963, 11, 463-471. DOI: https://doi.org/10.1016/S0021-9673(01)80947-X
Goldschmidt, S.; Renn, K. Ber. Dtsch. Chem. Ges. A. B. 1922, 55, 628-643. DOI: https://doi.org/10.1002/cber.19220550308
Osman, A.M. Biochem. Biophys. Res. Commun. 2011, 412, 473-478. DOI: https://doi.org/10.1016/j.bbrc.2011.07.123
Passari, A. K.; Leo, V. V.; Singh, G.; Samanta, L.; Ram, H.; Siddaiah, C. N.; Hashem, A.; Al-Arjani, A.-B. F.; Alqarawi, A. A.; Fathi Abd_Allah, E. Int. J. Mol. Sci. 2020, 21, 7364. DOI: https://doi.org/10.3390/ijms21197364
Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stri?ík, M.; Kowalczewski, P. ?.; Hlava?ková, L.; Rovná, K.; Žiarovská, J. Int. J. Mol. Sci. 2020, 21, 3087. DOI: https://doi.org/10.3390/ijms21093087
Mishra, K.; Ojha, H.; Chaudhury, N.K. Food Chem. 2012, 130, 1036-1043. DOI: https://doi.org/10.1016/j.foodchem.2011.07.127
Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Free Radicals Biol. Med. 1999, 26, 1231-1237. DOI: https://doi.org/10.1016/S0891-5849(98)00315-3
Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. J. Agric. Food Chem. 2004, 52, 7970-7981. DOI: https://doi.org/10.1021/jf048741x
Oyaizu, M. Jpn. J. Nutr. Diet. 1986, 44, 307-315. DOI : https://doi.org/10.5264/eiyogakuzashi.44.307
Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. Biochem. Pharmacol. 1961, 7, 88-95. DOI: https://doi.org/10.1016/0006-2952(61)90145-9
Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. Food Chem. 2013, 141, 2170-2176. DOI: https://doi.org/10.1016/j.foodchem.2013.04.123
Amrani, A.; Mecheri, A.; Bensouici, C.; Boubekri, N.; Benaissa, O.; Zama, D.; Benayache, F.; Benayache, S. Biocatal. Agric. Biotechnol. 2019, 20, 101209. DOI: https://doi.org/10.1016/j.bcab.2019.101209
Approved Standard-National Committee for Clinical Laboratory Standards Wayne, PA. 2008, 28, M27-A3.
Approved Standard-Tenth Edition; Document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA. 2015.
Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Mol. 2017, 22, 1382. DOI: https://doi.org/10.3390/molecules22081382
Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. J. Pharm. Anal. 2016, 6, 71-79. DOI: https://doi.org/10.1016/j.jpha.2015.11.005
Martínez-Francés, V.; Hahn, E.; Ríos, S.; Rivera, D.; Reich, E.; Vila, R.; Cañigueral, S. Front. Pharmacol. 2017, 8, 467. DOI: https://doi.org/10.3389/fphar.2017.00467
Mata, A.; Proença, C.; Ferreira, A.; Serralheiro, M.; Nogueira, J.; Araújo, M. Food Chem. 2007, 103, 778-786. DOI: https://doi.org/10.1016/j.foodchem.2006.09.017
Ruberto, G.; Baratta, M.T. Food Chem. 2000, 69,167-174. DOI: https://doi.org/10.1016/S0308-8146(99)00247-2
Loizzo, M.R.; Tundis, R.; Conforti, F.; Menichini, F.; Bonesi, M.; Nadjafi, F.; Frega, N.G.; Menichini, F. Nutr. Res. 2010, 30, 823-830. DOI: https://doi.org/10.1016/j.nutres.2010.09.016
Fu, Z.; Wang, H.; Hu, X.; Sun, Z.; Han, C. J. Appl. Pharm. Sci. 2013, 3, 122-127. DOI: 10.7324/JAPS.2013.3723
Vladimir-Knezevic, S.; Blazekovic, B.; Kindl, M.; Vladic, J.; Lower-Nedza, A.D.; Brantner, A.H. Mol. 2014, 19, 767-782. DOI: https://doi.org/10.3390/molecules19010767
Perry, N.S.; Houghton, P.J.; Jenner, P.; Keith, A.; Perry, E.K. Phytomedicine. 2002, 9, 48-51. DOI: https://doi.org/10.1078/0944-7113-00082
Savelev, S.U.; Okello, E.J.; Perry, E.K. Phytother. Res. 2004, 18, 315-324. DOI: https://doi.org/10.1002/ptr.1451
Duru, M.E.; Tel, G.; Öztürk, M.; Harmandar, M. Rec. Nat. Prod. 2012, 6, 175-179.
Salinas, M.; Bec, N.; Calva, J.; Ramírez, J.; Andrade, J.M.; Larroque, C.,; Vidari, G.; Armijos, C. Rec. Nat. Prod. 2020, 14, 276-285. DOI: http://doi.org/10.25135/rnp.164.19.07.1342 DOI: https://doi.org/10.25135/rnp.164.19.07.1342
Savelev, S.; Okello, E.; Perry, N.S.; Wilkins, R.M.; Perry, E.K. Pharmacol., Biochem. Behav. 2003, 75, 661-668. DOI: https://doi.org/10.1016/S0091-3057(03)00125-4
Loizzo, M.R.; Menichini, F.; Tundis, R.; Bonesi, M.; Conforti, F.; Nadjafi, F.; Statti, G.A.; Frega, N.G.; Menichini, F. J. Oleo Sci. 2009, 58, 443-446. DOI: https://doi.org/10.5650/jos.58.443
Bahadori, M.B.; Valizadeh, H.; Asghari, B.; Dinparast, L.; MoridiFarimani, M.; Bahadori, S. J. Funct. Foods. 2015, 18, 727-736. DOI:https://doi.org/10.1016/j.jff.2015.09.011
Burt, S. Int. J. Food Microbiol. 2004, 94, 223-253. DOI: https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
Mourey, A.; Canillac, N. Food Control. 2002, 13, 289-292. DOI: https://doi.org/10.1016/S0956-7135(02)00026-9
Koroch, A. R.; Juliani, H. R.; Zygadlo, J. A. Flavours Fragrances. 2007, 87-115. DOI: https://doi.org/10.1007/978-3-540-49339-6_5
Traoré, Y.; Ouattara, K.; Yéo, D.; Doumbia, I.; Coulibaly, A. J. Appl. Biosci. 2012, 58, 4234-4242.
Mastelic, J.; Politeo, O.; Jerkovic, I.; Radosevic, N. Chem. Nat. Compd. 2005, 41, 35-40. DOI: https://doi.org/10.1007/s10600-005-0069-z

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








