Synthesis of Coumarins Linked With 1,2,3-Triazoles under Microwave Irradiation and Evaluation of their Antimicrobial and Antioxidant Activity
DOI:
https://doi.org/10.29356/jmcs.v64i1.1116Keywords:
Coumarin, 1,2,3-triazole, click chemistry, antimicrobial, antioxidantAbstract
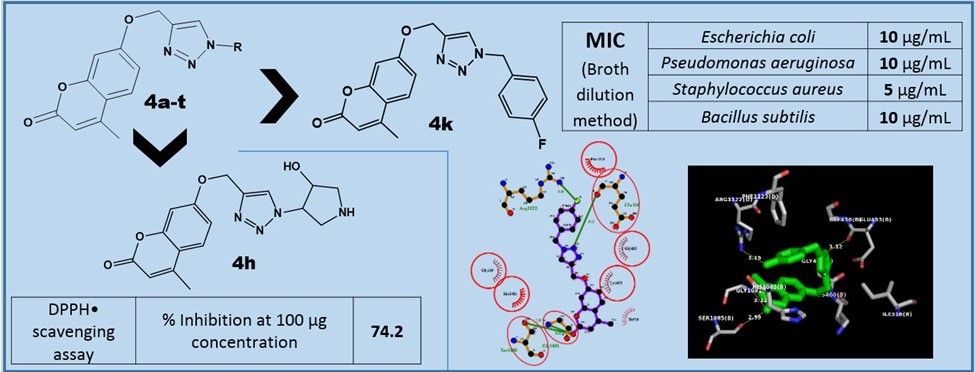
A series of coumarin derivatives linked with 1,2,3-triazoles has been synthesized by utilizing the copper catalyzed azide-alkyne cycloaddition reaction and were screened for their antimicrobial and antioxidant properties. Some of the compounds displayed promising antibacterial activities (MIC ranging from 5-150 µg/mL) and moderate antifungal activities as compared to the respective standards. The compounds 4k and 4g displayed good antibacterial activity when compared with the standard, Ciprofloxacin, and 4n exhibited better antifungal activity when compared to other synthesized compounds. The in silico docking studies of the active compounds were carried out against the gyrase enzyme and from those studies, it was acknowledged that 4k possessed significant hydrogen bonding and hydrophobic interactions which could be the plausible reason for its superior activity as compared to the other synthesized compounds. The compounds 4h and 4q showed promising antioxidant activity when compared with the standard, BHT, which could be attributed to the presence of electron donating substituents.
Resumen. Una serie de derivados de cumarina enlazados con 1,2,3-triazoles fue sintetizada empleando la reacción de cicloadición azida-alquino catalizada con cobre y fue evaluada en sus propiedades antimicrobianas y antioxidantes. Algunos de los compuestos exhibieron actividad antimicrobiana promisoria (intervalo MIC de 5-150 µg/mL) y actividad antifúngica moderada con respecto a los estándares respectivos. Los compuestos 4g y 4k mostraron buena actividad antibacterial con relación al estándar. Fluconazole y 4n exhibieron mejor actividad antifúngica en comparación con el resto de los compuestos. Se llevaron a cabo estudios in silico de modelado molecular e interacción de los compuestos activos con la enzima girasa ADN. De estos estudios se observó que 4k posee enlaces puentes de hidrógeno e interacciones hidrofóbicas significativos, los cuales podrían ser una causa plausible de su actividad mayor a aquélla mostrada por los otros compuestos sintetizados. Los compuestos 4h y 4q mostraron una importante actividad antioxidante comparada con el estándar (BHT), lo cual podría atribuirse a la presencia de sustituyentes electro-donadores
Downloads
References
Moellering, R. C. Am. J. Med. 1995, 99, 11S–18S. DOI: https://doi.org/10.1016/S0002-9343(99)80279-4
Singh, P.; Anand, A.; Kumar, V. Eur. J. Med. Chem. 2014, 85, 758–777. DOI: https://doi.org/10.1016/j.ejmech.2014.08.033
Peet, N. P. Drug. Discov. Today 2010, 15, 583–586. DOI: https://doi.org/10.1016/j.drudis.2010.04.002
Sugamura, Jr. K.; Keaney, J. F. Free Radical Biol. Med. 2011, 51, 978–992. DOI: https://doi.org/10.1016/j.freeradbiomed.2011.05.004
Yang, Y.; Liu, Q. W.; Shi, Y.; Song, Z. G.; Jin, Y. H.; Liu, Z. Q. Eur. J. Med. Chem. 2014, 84, 1–7. DOI: https://doi.org/10.1016/j.ejmech.2014.07.009
Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Curr. Med. Chem. 2005, 12, 887–916. DOI: https://doi.org/10.2174/0929867053507315
Al-Haiza, M. A.; Mostafa, M. S. Molecules 2003, 8, 275–286. DOI: https://doi.org/10.3390/80200275
Fylaktakidou, K. C.; Litina, D. H. J. Curr. Pharm. Des. 2004, 10, 3813–3833. DOI: https://doi.org/10.2174/1381612043382710
Lall, N.; Hussein, A. A.; Meyer, J. J. M. Fitoterapia 2006, 77, 230–232. DOI: https://doi.org/10.1016/j.fitote.2006.01.007
Kostova, I.; Raleva, S.; Genova, P.; Argirova, R. Bioinorg. Chem. Appl. 2006, 1–9. DOI: https://doi.org/10.1155/BCA/2006/68274
Hinman, J. W.; Hoeksema, H.; Caron, E. L.; Jackson, W. G. J. Am. Chem. Soc. 1956, 78, 1072–1074. DOI: https://doi.org/10.1021/ja01586a055
Kawaguchi, H.; Tsukiura, H.; Okanishi, M.; Miyaki, T.; Ohmori, T.; Fujisawa, K.; Koshiyama, H. J. Antibiotics, Ser A. 1965, 18, 1–10.
Kumbhare, R. M.; Kosurkar, U. B.; Ramaiah, M. J.; Dadmal, T. L.; Pushpavalli, S. N. C. V. L.; Pal-Bhadra, M. Bioorg. Med. Chem. Lett. 2012, 22, 5424–5427. DOI: https://doi.org/10.1016/j.bmcl.2012.07.041
Pawelec, G.; Ehninger, G.; Rehbein, A.; Schaudt, K.; Jaschonek, K. Int. J. Immunopharmacol. 1991, 13, 299–304. DOI: https://doi.org/10.1016/0192-0561(91)90111-J
Genin, M. J.; Allwine, D. A.; Anderson, D. J.; Barbachyn, M. R.; Emmert, D. E.; Garmon, S. A.; Graber, D. R.; Grega, K. C.; Hester, J. B.; Hutchinson, D. K.; Morris, J.; Reischer, R. J.; Ford, C. W.; Zurenco, G. E.; Hamel, J. C.; Schaadt, R. D.; Stapertand, D.; Yagi, B. H. J. Med. Chem. 2000, 43, 953–970. DOI: https://doi.org/10.1021/jm990373e
Jordao, A. K.; Afonso, P. P.; Ferreira, V. F.; de Souza, M. C.; Almeida, M. C.; Beltrame, C. O.; Paiva, D. P.; Wardell, S. M.; Wardell, J. L.; Tiekink, E. R.; Damaso, C. R.; Cunha, A. C. Eur. J. Med. Chem. 2009, 44, 3777–3783. DOI: https://doi.org/10.1016/j.ejmech.2009.04.046
Buckle, D. R.; Outred, D. J.; Rockell, C. J. M.; Smith, H.; Spicer, B. A. J. Med. Chem. 1983, 26, 251–254. DOI: https://doi.org/10.1021/jm00356a025
Hager, C.; Miethchen, R.; Reinke, H. J. Fluor. Chem. 2000, 104, 135–142. DOI: https://doi.org/10.1016/S0022-1139(00)00212-8
Kolb, H. C.; Sharpless, K. B. Drug. Discov. Today 2003, 8, 1128–1137. DOI: https://doi.org/10.1016/S1359-6446(03)02933-7
Singh, P.; Raj, R.; Kumar, V.; Mahajan, M. P.; Bedi, P. M. S.; Kaur, T.; Saxena, A. K. Eur. J. Med. Chem. 2012, 47, 594–600. DOI: https://doi.org/10.1016/j.ejmech.2011.10.033
Zhou, C. H.; Wang, Y. Curr. Med. Chem. 2012, 19, 239–280. DOI: https://doi.org/10.2174/092986712803414213
Kappe, C. O.; Dallinger, D. Mol. Diversity 2009, 13, 71–193. DOI: https://doi.org/10.1007/s11030-009-9138-8
Hoz, A.; Ortiz, A. D.; Moreno, A. Chem. Soc. Rev. 2005, 34, 164–178. DOI: https://doi.org/10.1039/B411438H
Meunier, B. Acc. Chem. Res. 2008, 41, 69–77. DOI: https://doi.org/10.1021/ar7000843
Tietze, L. F.; Bell, H. P.; Chandrasekhar, S. Angew. Chem. Int. Ed. 2003, 42, 3996–4028. DOI: https://doi.org/10.1002/anie.200200553
Li, W.T.; Wu, W.H.; Tang, C.H.; Tai, R.; Chen, S.T. ACS Comb. Sci. 2011, 13, 72–78. DOI: https://doi.org/10.1021/co1000234
Olesen, P. H.; Sørensen, A. R.; Ursø, B.; Kurtzhals, P.; Bowler, A. N.; Ehrbar, U.; Hansen, B. F. J. Med. Chem. 2003, 46, 3333?3341. DOI: https://doi.org/10.1021/jm021095d
Sajith, A. M.; Khader, K. K. A.; Joshi, N.; Reddy, M. N.; Padusha, M. S. A.; Nagaswarupa, H. P.; Joy, M. N.; Bodke, Y. D.; Karuvalam, R. P.; Banerjee, R.; Muralidharan, A.; Rajendra, P. Eur. J. Med. Chem. 2015, 89, 21–31. DOI: https://doi.org/10.1016/j.ejmech.2014.10.037
Joy, M. N.; Bakulev, V. A. AIP Conference Proceedings 2019, 2063, 030015. DOI: https://doi.org/10.1063/1.5087323
Savitha, B.; Reddy, E. K.; Kumar, C. S. A.; Karuvalam, R. P.; Padusha, M. S. A.; Bakulev, V. A.; Narasimhamurthy, K. H.; Sajith, A. M.; Joy, M. N. Tetrahedron Lett. 2019, 60, 151332. DOI: https://doi.org/10.1016/j.tetlet.2019.151332
Kenchappa, R.; Bodke, Y. D.; Aswathanarayanappa, C.; Telkar, S.; Manjunatha, K. S.; Sindhe, A. M. Ara. J. Chem. 2017, 10, S1336–S1344. DOI: https://doi.org/10.1016/j.arabjc.2013.03.020
Yadav, N.; Agarwal, D.; Kumar, S.; Dixit, A. K.; Gupta, R. D.; Awasthi, S. K. Eur. J. Med. Chem. 2018, 145, 735–745. DOI: https://doi.org/10.1016/j.ejmech.2018.01.017
Shaikh, M. H.; Subhedar, D. D.; Shingate, B. B.; Khan, F. A. K.; Sangshetti, J. N.; Khedkar, V. M.; Nawale, L.; Sarkar, D.; Navale, G. R.; Shinde, S. S. Med. Chem. Res. 2016, 25, 790–804. DOI: https://doi.org/10.1007/s00044-016-1519-9
Kumari, S.; Joshi, S.; Shakoor, S. M. A.; Agarwal, D. S.; Panda, S. S.; Pant, D. D.; Sakhuja, R. Aus. J. Chem. 2015, 68, 1415–1426. DOI: https://doi.org/10.1071/CH14708
Pramitha, P.; Bahulayan, D. Bioorg. Med. Chem. Lett. 2012, 22, 2598–2603. DOI: https://doi.org/10.1016/j.bmcl.2012.01.111
Potdar, M. K.; Mohile, S. S.; Salunkhe, M. M. Tetrahedron Lett. 2001, 42, 9285–9287. DOI: https://doi.org/10.1016/S0040-4039(01)02041-X
Sheikh, J.; Parvez, A.; Juneja, H.; Ingle, V.; Chohan, Z.; Youssoufi, M.; Hadda, T. B. Eur. J. Med. Chem. 2011, 46, 1390–1399. DOI: https://doi.org/10.1016/j.ejmech.2011.01.068
Alonso, R.; Fernandez-Aranquiz, A.; Colom, K.; Herreras, A.; Cisterna, R. Rev. Esp. Quimioter. 2000, 13, 384–393.
Sader, H. S.; Jones, R. N.; Silva, J. B. Diagn. Microbiol. Infect. Dis. 2002, 44, 281–288. DOI: https://doi.org/10.1016/S0732-8893(02)00468-6
Parker, M. A.; Kurrasch, D. M.; Nichols, D. E. Bioorg. Med. Chem. 2008, 16, 4661–4669. DOI: https://doi.org/10.1016/j.bmc.2008.02.033
Niki, E. Chem. Phys. Lipids 1987, 44, 227–253. DOI: https://doi.org/10.1016/0009-3084(87)90052-1
Matos, M. J.; Pérez-Cruz, F.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L.; Borges, F.; Olea-Azar, C. Bioorg. Med. Chem. 2013, 21, 3900–3906. DOI: https://doi.org/10.1016/j.bmc.2013.04.015
Yamagami, C.; Akamatsu, M.; Motohashi, N.; Hamada, S.; Tanahashi, T. Bioorg. Med. Chem. Lett. 2005, 15, 2845–2850. DOI: https://doi.org/10.1016/j.bmcl.2005.03.087
Sivakumar, P. M.; Prabhakar, P. K.; Doble, M. Med. Chem. Res. 2011, 20, 482–492. DOI: https://doi.org/10.1007/s00044-010-9342-1
Wigley, D. B.; Davies, G. J.; Dodson E. J.; Maxwell, A.; Dodson, G. Nature 1991, 351, 624–629. DOI: https://doi.org/10.1038/351624a0
Cabral, J. H. M.; Jackson, A. P.; Smith, C. V.; Shikotra, N.; Maxwell, A.; Liddington, R. C. Nature 1997, 388, 903–906. DOI: https://doi.org/10.1038/42294
Bradbury, B. J.; Pucci, M. J. Curr. Opin. Pharmacol. 2008, 8, 574–581. DOI: https://doi.org/10.1016/j.coph.2008.04.009
Ehmann, D. E.; Lahiri, S. D. Curr. Opin. Pharmacol. 2014, 18, 76–83. DOI: https://doi.org/10.1016/j.coph.2014.09.007
Tse-Dinh, Y. C. Infect. Disord. Drug Targets 2007, 7, 3–9. DOI: https://doi.org/10.2174/187152607780090748
Collin, F.; Karkare, S.; Maxwell, A. Appl. Microbiol. Biotechnol. 2011, 92, 479–497. DOI: https://doi.org/10.1007/s00253-011-3557-z
http://www.citeulike.org/group/340/article/240061 accessed in October 2014.
Sander, T.; Freyss, J.; Korff, M. V.; Reich, J. R.; Rufener, C. J. Chem. Inf. Model 2009, 49, 232–246. DOI: https://doi.org/10.1021/ci800305f
Trott, O.; Olson, A. J. J. Comput. Chem. 2010, 31, 455–461. DOI: https://doi.org/10.1002/jcc.21334
Bax, B. D.; Chan, P. F.; Eggleston, D. S.; Fosberry, A.; Gentry, D. R.; Gorrec, F. Nature 2010, 466, 935–940. DOI: https://doi.org/10.1038/nature09197
Morris, G. M.; Goodsell, D. S.; Halliday, R. S.; Huey, R.; Hart, W. E.; Belew, R. K. J. Comput. Chem. 1998, 19, 1639–1662. DOI: https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
Laskowski, R. A.; Swindells, M. B. J. Chem. Inf. Model 2011, 51, 2778–2786. DOI: https://doi.org/10.1021/ci200227u

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









