In silico Exploration of Leads from Lichen Derived Salazinic Acid, Sekikaic Acid and Usnic Acid Targeting HER2 in Breast Cancer
DOI:
https://doi.org/10.29356/jmcs.v69i4.2175Keywords:
Breast cancer, usnic acid, HER2, docking, PASS predictionAbstract
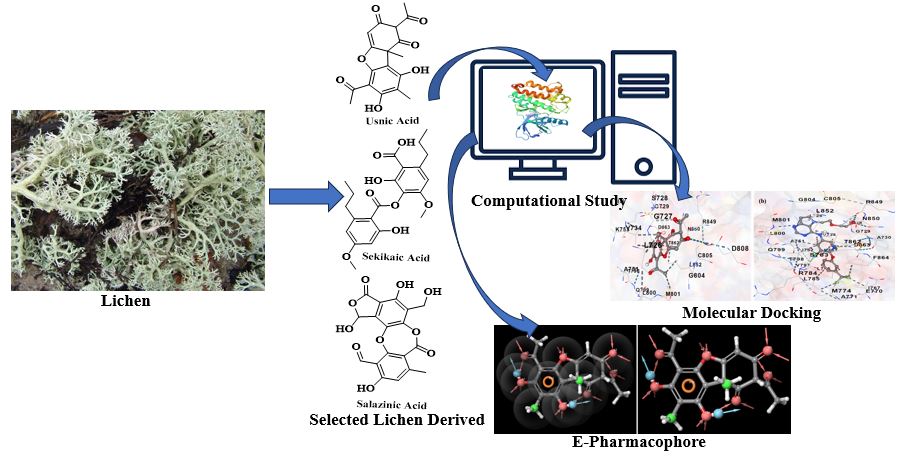
Abstract. One of the most common cancers that strikes women is breast cancer (BC). Twenty percent of cases of BC are caused by human epidermal growth factor receptor-2 (HER2), which may be a target for the development of BC medicines. Consequently, the main goal was to find a BC inhibitor by using pass prediction and in silico docking techniques. Usnic acid may be used as a potential HER2 inhibitor, according to in silico study results, and compounds with high binding free energies may have significant anti-BC effects, making them promising candidates for further therapeutic development. Usnic acid was shown to have an inhibitory effect against HER2 of -8.9 kcal/mol, which was comparable to the reference substance (co-crystal; -9.7 kcal/mol). Additionally, because the probability active (Pa) value of usnic acid is greater than 0.700, it possesses a broad spectrum of anti-neoplastic properties against BC. The main substance in the present study that can suppress BC has been shown to be usnic acid, an active lichen extract. The present computational findings will be validated in a wet lab using both in vitro and in vivo tests.
Resumen. Uno de los cánceres más comunes que afecta a las mujeres es el cáncer de mama (CM). El veinte por ciento de los casos de BC son causados por el receptor 2 del factor de crecimiento epidérmico humano (HER2), que puede ser un objetivo para el desarrollo de medicamentos contra la BC. En consecuencia, el objetivo principal era encontrar un inhibidor de BC mediante el uso de predicción de pases y técnicas de acoplamiento in silico. El ácido úsnico puede usarse como un posible inhibidor de HER2, según los resultados de un estudio in silico, y los compuestos con altas energías libres de unión pueden tener importantes efectos anti-BC, lo que los convierte en candidatos prometedores para un mayor desarrollo terapéutico. Se demostró que el ácido úsnico tiene un efecto inhibidor contra HER2 de -8.9 kcal/mol, comparable al de la sustancia de referencia (cocristal; -9.7 kcal/mol). Además, debido a que el valor de probabilidad activa (Pa) del ácido úsnico es superior a 0.700, posee un amplio espectro de propiedades antineoplásicas contra BC. Se ha demostrado que la principal sustancia en el presente estudio que puede suprimir la BC es el ácido úsnico, un extracto activo de liquen. Los presentes hallazgos computacionales se validarán en un laboratorio húmedo mediante pruebas tanto in vitro como in vivo.
Downloads
References
1. Sohrab, S.S.; Kamal, M.A. Life. 2022, 12, 1729.
2. Kolak, A.; Kamińska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukiełka-Budny, B.; Burdan, F. Ann. Agric. Environ. Med. 2017, 24.
3. Ottini, L.; Capalbo, C.; Rizzolo, P.; Silvestri, V.; Bronte, G.; Rizzo, S.; Russo, A. Breast Cancer: Targets Ther. 2010, 45-58.
4. Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Science. 1987, 235, 177-182.
5. Tarantino, P.; Curigliano, G.; Tolaney, S.M. Cancer Discovery. 2022, 12, 2026-2030.
6. Baselga, J.; Swain, S.M. Nat. Rev. Cancer. 2009, 9, 463-475.
7. Shams ul Hassan, S.; Abbas, S.Q.; Hassan, M.; Jin, H.Z. Anti-Cancer Agents Med. Chem. 2022, 22, 731-746.
8. Candan, M.; Yılmaz, M.; Tay, T.; Erdem, M.; Türk, A.Ö. Zeitschrift für Naturforschung C. 2007, 62, 619-621.
9. Bhattacharyya, S.; Deep, P.R.; Singh, S.; Nayak, B. Am. J. PharmTech Res. 2016, 6, 1-7.
10. Verma, N.; Behera, B.C.; Sharma, B.O. Hacettepe Journal of Biology and Chemistry. 2012, 40, 7-21.
11. Morris Kupchan, S.; Kopperman, H.L. Experientia. 1975, 31, 625-625.
12. Roney, M.; Singh, G.; Huq, A.M.; Forid, M.S.; Ishak, W.M.B.W.; Rullah, K.; Tajuddin, S.N. Mol. Biotechnol. 2023, 1-11.
13. Aertgeerts, K.; Skene, R.; Yano, J.; Sang, B.C.; Zou, H.; Snell, G.; Sogabe, S. J. Biol. Chem. 2011, 286, 18756-18765.
14. Liu, Y.; Grimm, M.; Dai, W.T.; Hou, M.C.; Xiao, Z.X.; Cao, Y. Acta Pharmacol. Sin. 2020, 41, 138-144.
15. Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Chem. Heterocycl. Compd. 2014, 50, 444-457.
16. Qais, F.A.; Alomar, S.Y.; Imran, M.A.; Hashmi, M.A. Molecules. 2022, 27, 5793.
17. Rani, A.C.; Kalaimathi, K.; Jayasree, S.; Prabhu, S.; Vijayakumar, S.; Ramasubbu, R.; Priya, N.S. Chem. Afr. 2022, 1-14.
18. Kiliç, N.; Islakoğlu, Y.Ö.; Büyük, İ.; Gür-Dedeoğlu, B.; Cansaran-Duman, D. D. Anti-Cancer Agents Med. Chem. 2019, 19, 1463-1472.
19. Özben, R.Ş.; Cansaran-Duman, D. Human Experimen. Toxicol. 2020, 39, 1497-1506.
20. Cansaran-Duman, D.; Tanman, Ü.; Yangın, S.; Atakol, O. Cytotechnol. 2020, 72, 855-872.
21. Zuo, S.T.; Wang, L.P.; Zhang, Y.; Zhao, D.N.; Li, Q.S.; Shao, D.; Fang, X.D. RSC Adv. 2015, 5, 153-162.
22. Pyrczak-Felczykowska, A.; Reekie, T.A.; Jąkalski, M.; Hać, A.; Malinowska, M.; Pawlik, A.; Herman-Antosiewicz, A. Int. J. Mol. Sci. 2022, 23, 1802.
23. Wong, K.K.V.; Roney, M.; Uddin, N.; Imran, S.; Gazali, A.M.; Zamri, N.; Aluwi, M.F.F.M. J. Biomol. Struct. Dyn. 2023, 1-14.
24. Zuo, S.T.; Wang, L.P.; Zhang, Y.; Zhao, D.N.; Li, Q.S.; Shao, D.; Fang, X.D. RSC Advances. 2015, 5, 153-162.
25. Song, Y.; Dai, F.; Zhai, D.; Dong, Y.; Zhang, J.; Lu, B.; Yi, Z. Angiogenesis. 2012, 15, 421-432.
26. Roney, M.; Issahaku, A.R.; Govinden, U.; Gazali, A.M.; Aluwi, M.F.F.M.; Zamri, N.B. J. Biomol. Struct. Dyn. 2023, 1-14.
27. https://www.who.int/news-room/fact-sheets/detail/breast-cancer, accessed in August 2025.
28. Cordero, M.J.A.; Villar, N.M.; Sánchez, M.N.; Pimentel-Ramírez, M.L.; García-Rillo, A.; Valverde, E.G. Nutr. Hosp. 2015, 31, 371-379.
29. Al-Khodairy, F.M.; Khan, M.K.A.; Kunhi, M.; Pulicat, M.S.; Akhtar, S.; Arif, J.M. Am. J. Bioinform. Res. 2013, 3, 62-71.
30. Yousuf, Z.; Iman, K.; Iftikhar, N.; Mirza, M.U. Breast Cancer: Targets Ther. 2017, 447-459.
31. Ashtekar, S.S.; Bhatia, N.M.; Bhatia, M.S. Int. J. Pept. Res. Ther. 2019, 25, 659-667.
32. Muhammad, A.; Katsayal, B.S.; Forcados, G.E.; Malami, I.; Abubakar, I.B.; Kandi, A.I.; Umar, Z.W.S. In Silico Pharmacology. 2020, 8, 1-13.
33. Shrihastini, V.; Muthuramalingam, P.; Adarshan, S.; Sujitha, M.; Chen, J.T.; Shin, H.; Ramesh, M. Cancers. 2021, 13, 6222.
34. Tripathi, A.H.; Negi, N.; Gahtori, R.; Kumari, A.; Joshi, P.; Tewari, L.M.; Upadhyay, S.K. Anti-Cancer Agents Med. Chem. 2022, 22, 115-142.
35. Nguyen, T.T.; Yoon, S.; Yang, Y.; Lee, H.B.; Oh, S.; Jeong, M.H.; Kim, H. PloS one. 2014, 9, e111575.
36. Opo, F.A.D.M.; Rahman, M.M.; Ahammad, F.; Ahmed, I.; Bhuiyan, M.A.; Asiri, A.M. Sci. Rep. 2021, 11, 4049.
37. Uddin, M.J.; Ali Reza, A.S.M.; Abdullah-Al-Mamun, M.; Kabir, M.S.; Nasrin, M.S.; Akhter, S.; Rahman, M.A. Front. Pharmacol. 2018, 9, 246.
38. James, N.; Surana, R.; Thigale, I.; Preethi, B.; Shanthi, V.; Ramanathan, K. Indian J. Pharm. Educ. Res. 2018, 52, 707-717.
39. Roney, M.; Huq, A.M.; Rullah, K.; Hamid, H.A.; Imran, S.; Islam, M.A.; Mohd Aluwi, M.F.F. J. Comput. Biophys. Chem. 2021, 20, 797-814.

Downloads
Published
Issue
Section
License
Copyright (c) 2025 Miah Roney, Amit Dubey, Mohd Fadhlizil Fasihi Mohd Aluwi

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









