CaCO₃ (Calcite) Co-Doped with Eu and Cr: The Effect of Chromium/Europium Ratio on Blue Light Emission
DOI:
https://doi.org/10.29356/jmcs.v69i3.2185Keywords:
CaCO3: Eu-Cr, light emission, blueshiftAbstract
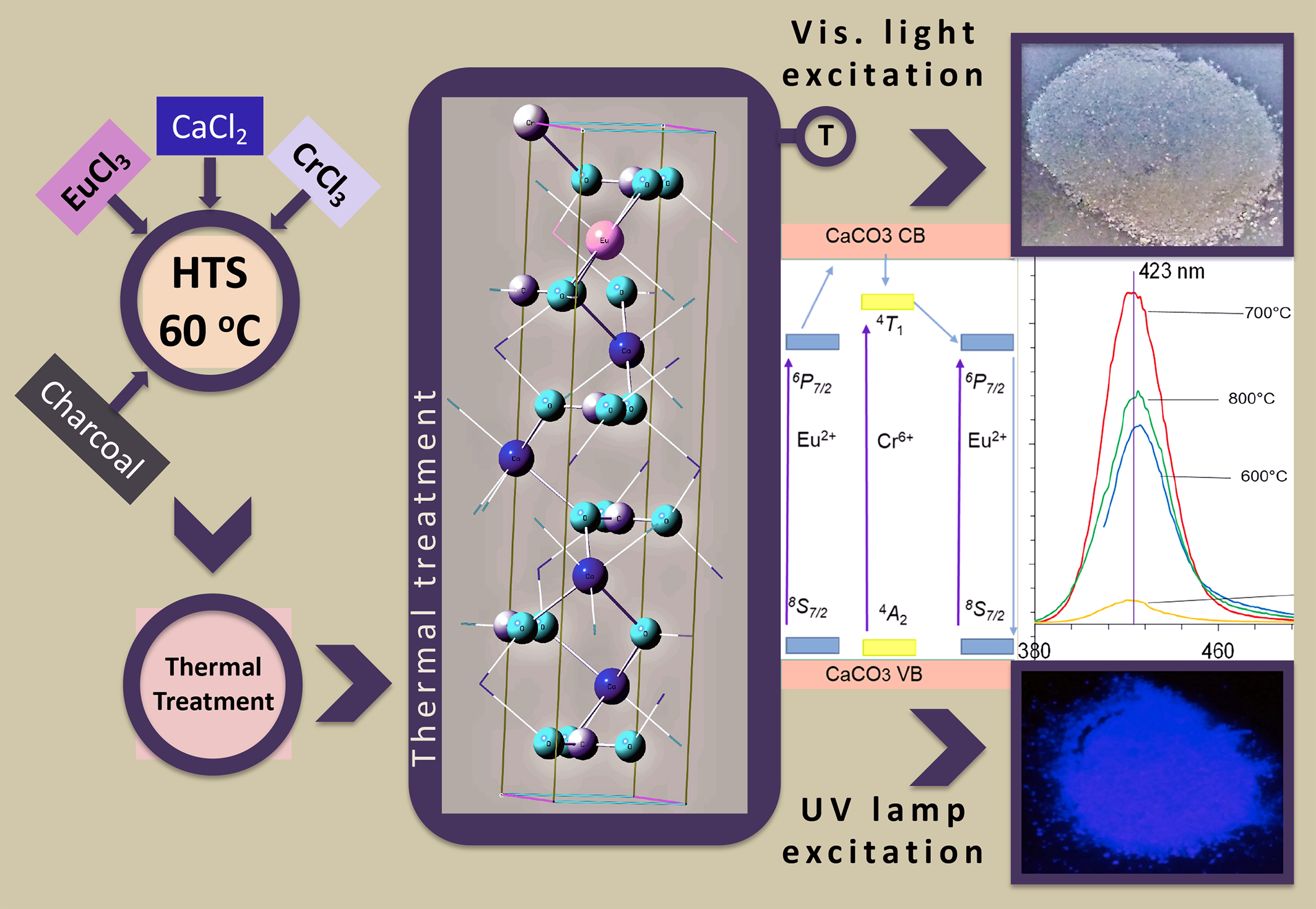
Abstract. The emission spectra of CaCO3 co-doped with Eu and Cr have been studied in the UV-Vis region. A change in both the emission wavelength from 426 to 423 nm and the intensity, which is 30 times higher with respect to CaCO3:Eu, indicates a significant modification of the optical properties of CaCO3:Eu due to the influence of chromium (Cr/Eu = 0.5). Changes in the band gap (Eg) from 6 to 5.2 eV were observed when the material was calcined at 700 °C. This emission phenomenon was also observed at 600, 800 and 900 °C, but with lower intensity. Analysis of the excitation and emission bands indicates that Eu is in the 2+ oxidation state and Cr is in the 6+ oxidation state. This is because the emission occurs in the blue-violet region of the visible spectrum when exposed to UV light with a wavelength of 254 nm. The material has potential LED applications.
Resumen. Se estudiaron los espectros de emisión del CaCO3 codopado con Eu y Cr en la región UV-Vis. Se observaron cambios en la longitud de onda de emisión de 426 a 423 nm y en la intensidad que resultó 30 veces mayor respecto a CaCO3:Eu, lo que indica una modificación significativa en las propiedades ópticas de CaCO3:Eu debido a la influencia del cromo (Cr/Eu = 0.5). Se observaron cambios en la energía de brecha (Eg) de 6 a 5.2 eV cuando el material se calcinó a 700 °C. Este fenómeno de emisión se observó también a 600, 800 y 900 °C, aunque con menor intensidad. El análisis de las bandas de excitación y emisión indica que el europio se encuentra en estado de oxidación 2+ y el cromo en estado de oxidación 6+. Esto se debe a que la emisión se produce en la región azul-violeta del espectro visible cuando se expone a una luz UV con una longitud de onda de 254 nm, por lo que el material puede tener aplicaciones potenciales como LED.
Downloads
References
Li, W. M.; Hänninen, T.; Leskelä, M.; Saari, J.; Hase, A. J. Alloy. Compd. 2001, 323-324, 236–238. DOI: http://dx.doi.org/10.1016/s0925-8388(01)01050-7. DOI: https://doi.org/10.1016/S0925-8388(01)01050-7
Weir, C. E.; Lippincott, E. R. J. Res. NBS. A Phys. Ch. 1961, 65, 173. DOI: http://dx.doi.org/10.6028/jres.065a.021. DOI: https://doi.org/10.6028/jres.065A.021
Hossain, F. M.; Murch, G. E.; Belova, I. V.; Turner, B. D. Solid State Commun. 2009, 149, 1201–1203. DOI: http://dx.doi.org/10.1016/j.ssc.2009.04.026. DOI: https://doi.org/10.1016/j.ssc.2009.04.026
Kowalska, K.; Kuwik, M.; Polak, J.; Pisarska, J.; Pisarski, W. A. Materials. 2021, 14, 7156. DOI: http://dx.doi.org/10.3390/ma14237156. DOI: https://doi.org/10.3390/ma14237156
Zhou, B.; Liu, B.; Zou, H.; Song, Y.; Gong, L.; Huo, Q.; Xu, X.; Sheng, Y. Colloids Surf., A. 2014, 447, 166–171. DOI: http://dx.doi.org/10.1016/j.colsurfa.2013.12.078. DOI: https://doi.org/10.1016/j.colsurfa.2013.12.078
Tamatani, M., in: Principal phosphor materials and their optical properties, 2nd ed.; Phosphor Handbook, CRC/Taylor and Francis, 2007, 178–182.
López-Arce, P.; Gómez-Villalba, L.S.; Martínez-Ramírez, S.; Álvarez de Buergo, M.; Fort, R. Powder Technol., 2010, 205, 263–269. DOI: http://dx.doi.org/10.1016/j.powtec.2010.09.026. DOI: https://doi.org/10.1016/j.powtec.2010.09.026
Graf, D. L. Am. Mineral. 1961, 46, 11-12. DOI: https://doi.org/10.1353/swe.1961.a962701
Dove, M. T.; Powell, B. M. Phys. Chem. Miner. 1989, 16. DOI: http://dx.doi.org/10.1007/bf00197019. DOI: https://doi.org/10.1007/BF00197019
Ishizawa, N.; Setoguchi, H.; Yanagisawa, K. Sci Rep-UK. 2013, 3. DOI: http://dx.doi.org/10.1038/srep02832. DOI: https://doi.org/10.1038/srep02832
Dorenbos, P. J. Lumin. 2000, 91, 91–106. DOI: http://dx.doi.org/10.1016/s0022-2313(00)00197-6. DOI: https://doi.org/10.1016/S0022-2313(00)00197-6
Dorenbos, P. J. Lumin. 2003, 104, 239–260. DOI: http://dx.doi.org/10.1016/s0022-2313(03)00078-4. DOI: https://doi.org/10.1016/S0022-2313(03)00078-4
Dorenbos, P. J. Phys-Condensed Mat. 2003, 15, 575–594. DOI: http://dx.doi.org/10.1088/0953-8984/15/3/322. DOI: https://doi.org/10.1088/0953-8984/15/3/322
Tanguy, B.; Merle, P.; Pezat, M.; Fouassier, C. Mater. Res. Bull. 1974, 9, 831–836. DOI: http://dx.doi.org/10.1016/0025-5408(74)90119-6. DOI: https://doi.org/10.1016/0025-5408(74)90119-6
Aboshyan-Sorgho, L.; Cantuel, M.; Petoud, S.; Hauser, A.; Piguet, C. Coordination Chem. Rev. 2012, 256, 1644–1663. DOI: http://dx.doi.org/10.1016/j.ccr.2011.12.013. DOI: https://doi.org/10.1016/j.ccr.2011.12.013
Wang, D.; Zhang, X.; Wang, X.; Leng, Z.; Yang, Q.; Ji, W.; Lin, H.; Zeng, F.; Li, C.; Su, Z. Crystals. 2020, 10, 1019. DOI: http://dx.doi.org/10.3390/cryst10111019. DOI: https://doi.org/10.3390/cryst10111019
Barton, D. G.; Soled, S. L.; Iglesia, E. J. Phys. Chem. 1999, 6, 87–99. DOI: http://dx.doi.org/10.1023/a:1019126708945. DOI: https://doi.org/10.1023/A:1019126708945
Medeiros, S. K.; Albuquerque, E. L.; Maia, F. F.; Caetano, E. W. S.; Freire, V. N. J. Phys. D Appl. Phys. 2007, 40, 5747–5752. DOI: http://dx.doi.org/10.1088/0022-3727/40/18/036. DOI: https://doi.org/10.1088/0022-3727/40/18/036
Atkins, P. W.; Shriver, D. E.; Overton, T.; Rourke, J.; Weller, M.; Armstrong, F., in: Inorganic Chemistry, 4th ed.; McGraw Hill, 2008, 19, 473.
http://wwwchem.uwimona.edu.jm/lab_manuals/CrTSexptnu/TSd3.html, accessed in August 2022.
Adachi, C.; Tsutsui, T., in: Fundamentals of luminescence, 2nd ed.; Phosphor Handbook, CRC/Taylor and Francis, 2007, 66–69.
Nassau, K., in: Color for Science, Art and Technology (AZimuth). North Holland, 1997, 45–50.
Caldiño, U.; Lira, A.; Meza-Rocha, A. N.; Camarillo, I.; Lozada-Morales, R. J. Lumin. 2018, 194, 231–239. DOI: http://dx.doi.org/10.1016/j.jlumin.2017.10.028. DOI: https://doi.org/10.1016/j.jlumin.2017.10.028
Bünzli, J. C. G. Handb. Phys. Chem. Rare Earths. 2016, 50, 141–176. DOI: http://dx.doi.org/10.1016/bs.hpcre.2016.08.003. DOI: https://doi.org/10.1016/bs.hpcre.2016.08.003
Yen, W.M.; Shionoya, S.; Yamamoto, H., in: Phosphor Handbook. CRC Press/Taylor and Francis, 2007, 149–151. DOI: https://doi.org/10.1201/9781420005233

Downloads
Published
Issue
Section
License
Copyright (c) 2025 E. E. Cervantes-Trujillo, S. A. Gómez-Torres, U. Caldiño, V. H. Lara-Corona

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









