Synthesis, in vitro Antitrichomonal Activity, and Docking Study of N-[(4-substituted phenyl)-1,3-thiazol-2-yl]-4-substituted Benzenesulfonamides
DOI:
https://doi.org/10.29356/jmcs.v68i1.1975Keywords:
Phenylthiazolyl benzenesulfonamide, Phenylthiazole, Benzenesulfonamide, Trichomonas vaginalis, Trichomoniasis, Docking studies, Trichomonas vaginalis ferredoxinAbstract
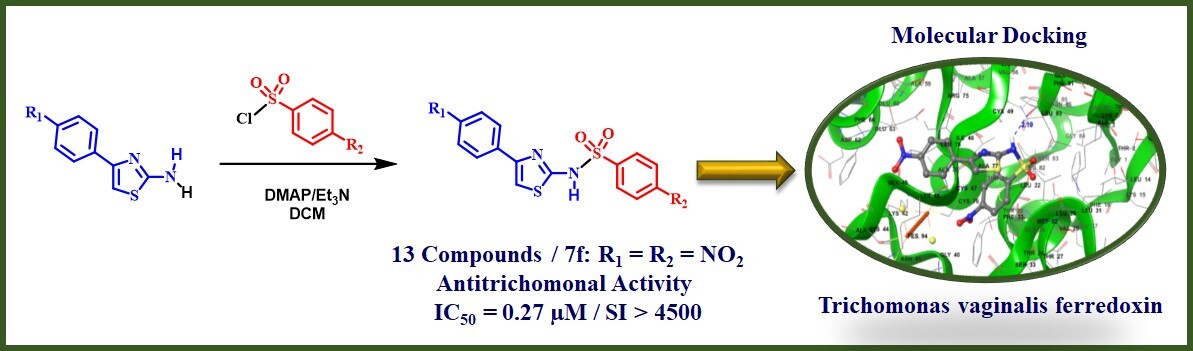
Infection by Trichomonas vaginalis has a high incidence/prevalence worldwide. It has been associated with a predisposition to cervical neoplasia or prostate cancer and an increased risk of acquisition of human papillomavirus (HPV) and human immunodeficiency virus (HIV). Besides, resistance to the drugs used for trichomoniasis treatment has increased in the last 30 years. Herein, thirteen phenylthiazolylbenzene sulfonamides were synthesized and evaluated for their in vitro activity against Trichomonas vaginalis. Among them, four derivatives showed higher anti-trichomonal activity than metronidazole (IC50 = 0.93 µM), while their cytotoxicity levels were not significant. These compounds were subject to molecular docking studies using Trichomonas vaginalis ferredoxin as target. The results revealed that the orientation of the nitro group of the active derivatives is toward [2Fe-2S], the cluster responsible for high reactive oxygen species generation. Finally, it was evident that the presence of a nitro group in the structure of the synthesized phenylthiazolylbenzene sulfonamides is essential for their trichomonicidal activity.
Resumen. A nivel mundial la infección por Trichomonas vaginalis tiene una alta incidencia/prevalencia y se ha asociado con una predisposición a padecer neoplasia cervical o cáncer de próstata, así como a generar un mayor riesgo de adquirir el virus del papiloma humano (VPH) y el virus de la inmunodeficiencia humana (VIH). Además, en los últimos 30 años, la resistencia a los fármacos utilizados para el tratamiento de la tricomoniasis ha aumentado. En el presente trabajo, trece sulfonamidas de feniltiazolilbenceno fueron sintetizadas y evaluadas in vitro contra Trichomonas vaginalis. Cuatro de ellas exhibieron una actividad anti-tricomonas mayor que el metronidazol (CI50 = 0.93 µM), a la vez que citotoxicidad no significativa. Por tal motivo, estos compuestos fueron sometidos a estudios de acoplamiento molecular utilizando como diana a la ferredoxina de T. vaginalis. Los resultados revelaron que la orientación del grupo nitro de los derivados activos está dirigida hacia el grupo [2Fe-2S], responsable de la generación de especies de oxígeno altamente reactivas. Finalmente, se evidenció que la presencia de al menos un grupo nitro en la estructura de las sulfonamidas de feniltiazolilbenceno sintetizadas es esencial para su actividad tricomonicida.
Downloads
References
Capela, R.; Moreira, R.; Lopes, F. Int. J. Mol. Sci. 2019, 20, 5748. DOI: https://doi.org/10.3390/ijms20225748.
Garrido-Cardenas, J.A.; Mesa-Valle, C.; Manzano-Agugliaro F. Parasitology. 2018, 145, 699-712. DOI: https://doi.org/10.1017/S0031182017001718.
Rowley J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; Thwin, S.S.; Broutet, N.; Taylor, M.M. Bull. World Health Organ. 2019, 97, 548-562. DOI: https://doi.org/10.2471/BLT.18.228486.
Margarita, V.; Fiori, P.L.; Rappelli, P. Front. Cell. Infect. Microbiol. 2020, 10, 179. DOI: https://doi.org/10.3389/fcimb.2020.00179.
Stark, J.R.; Judson, G.; Alderete, J.F.; Mundodi, V.; Kucknoor, A.S.; Giovannucci, E.L.; Platz, E.A.; Sutcliffe, S.; Fall, K.; Kurth, T.; Ma, J.; Stampfer, M.J.; Mucci, L.A. J. Natl. Cancer Inst. 2009, 101, 1406-1411. DOI: https://doi.org/10.1093/jnci/djp306.
Pasupuleti, V.; Escobedo, A.A.; Deshpande, A.; Thota, P.; Roman, Y.; Hernandez, A.V. PLoS Negl. Trop. Dis. 2014, 8, e2733.DOI: https://doi.org/10.1371/journal.pntd.0002733.
Grossman III, J.H.; Galask, R.P. Obstet. Gynecol. 1990, 76, 521-522. PMID: 2381638.
Bala, V.; Chhonker, Y.S. Eur. J. Med. Chem. 2018, 143, 232-243. DOI: https://doi.org/10.1016/j.ejmech.2017.11.029.
Borcea, A.M.; Ionut, I.; Crisan, O.; Oniga, O. Molecules. 2021, 26, 624. DOI: https://doi.org/10.3390/molecules26030624.
Mocelo-Castell, R.; Villanueva-Novelo, C.; Caceres-Castillo, D.; Carballo, R.M.; Quijano-Quinones, R.F.; Quesadas-Rojas, M.; Cantillo-Ciau, Z.; Cedillo-Rivera, R.; Moo-Puc, R.E.; Moujir, L.M.; Mena-Rejon, G. J. Open Chem. 2015, 13, 1127-1136. DOI: https://doi.org/10.1515/chem-2015-0127.
Mena-Rejón, G.; Pérez-Navarro, Y.; Torres-Romero, J.C.; Vázquez-Carrillo, L.; Carballo, R.M.; Arreola, R.; Herrera-España, A.; Arana-Argáez, V.; Quijano-Quiñones, R.; Fernández-Sánchez, J.M.; Alvarez-Sánchez, M.E. Parasitol. Res. 2021, 120, 233-241. DOI: https://doi.org/10.1007/s00436-020-06931-w.
Apaydın, S.; Török, M. Bioorg. Med. Chem. Lett. 2019, 29, 2042-2050. DOI: https://doi.org/10.1016/j.bmcl.2019.06.041.
Malagoli, M.; Rossi, T.; Baggio, A.; Zandomeneghi, G.; Zanca, A.; Casolari, C.; Castelli, M. Pharmacol. Res. 2002, 46, 469-472. DOI: https://doi.org/10.1080/14756366.2020.1863958.
Hernández-Núñez, E.; Tlahuext, H.; Moo-Puc, R.; Torres-Gómez, H.; Reyes-Martínez, R.; Cedillo-Rivera, R.; Nava-Zuazo, C.; Navarrete-Vazquez, G. Eur. J. Med. Chem. 2009, 44, 2975-2984. DOI: https://doi.org/10.1016/j.ejmech.2009.01.005.
Cedillo-Rivera, R.; Chávez, B.; González-Robles, A.; Tapia-Contreras, A.; Yépez-Mulia, L. J. Euk. Microbiol. 2002, 49, 201-208. DOI: https://doi.org/10.1111/j.1550-7408.2002.tb00523.x.
Rahman, A.; Choudhary, M.I.; Thomsen, W.J. Bioassay techniques for drug development. In: Manual of bioassay techniques for natural products research. Harwood Academic Publishers Netherlands, 2001. ISBN: 0-203-34349-2.
Lee, C.; Yang, W.; Parr, R.G. Phys. Rev. B. 1988, 37, 785-789. DOI: https://doi.org/10.1103/physrevb.37.785.
Spartan´20 Wavefunction, Inc., Irvine, CA.
Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. Nucleic. Acids Res. 2000, 28, 235-242. DOI: https://doi.org/10.1093/nar/28.1.235.
Morris, G. M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. J. Comput. Chem. 2009, 30. 2785-2791. DOI: https://doi.org/10.1002/jcc.21256.
Maestro, Schrödinger, LLC, New York, NY, 2019.
Caceres-Castillo, D.; Carballo, R.M.; Tzec-Interian, J.A.; Mena-Rejon, G. J. Tetrahedron Lett. 2012, 53, 3934-3936. DOI: https://doi.org/10.1016/j.tetlet.2012.05.093.
Carballo, R.M.; Padilla-Montano, N.; Reyes-Martinez, R.; Miron-Lopez G.; Hernandez-Ortega, S. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2014, 70, o6-7. DOI: https://doi.org/10.1107/S1600536814002086. DOI: https://doi.org/10.1107/S1600536813032145
Greenfield, A.; Grosanu, C. Tetrahedron Lett. 2008, 43, 6300-6303. DOI: https://doi.org/10.1016/j.tetlet.2008.08.054.
Röver, S.; Cesura, A.M.; Huguenin, P.; Kettler, R.; Szente, A. J. Med. Chem. 1997, 40, 4378-4385. DOI: https://doi.org/10.1021/jm970467t.
Hitchin, J.R.; Blagg, J.; Burke, R.; Burns, S.; Cockerill, M.J., Fairweather, E.E.; Hutton, C.; Jordan, A.M.; McAndrew, C.; Mirza, A.; Mould, D.; Thomson, G.J.; Waddella, I.; Ogilviea, D.J. MedChemComm. 2013, 4, 1513-1522. DOI: https://doi.org/10.1039/C3MD00226H.
Keseru, G.M.; Makara, G.M. Drug Discov. Today. 2006, 11, 741-748. DOI: https://doi.org/10.1016/j.drudis.2006.06.016.
Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. J. Ethnopharmacol. 2006, 106, 290-302. DOI: https://doi.org/10.1016/j.jep.2006.04.003.
Pink, R.; Hudson, A.; Mouri`es, M.-A; Bendig, M. Nat. Rev. Drug Discov. 2005, 4, 727-740. DOI: https://doi.org/10.1038/nrd1824.
Čėnas, N.; Nemeikaitė-Čėnienė, A.; Kosychova, L. Int. J. Mol. Sci. 2021, 22, 8534. DOI: https://doi.org/10.3390/ijms22168534.
Crossnoe, C.R.; Germanas, J.P.; LeMagueres, P.; Mustata, G.; Krause, K.L. J. Mol. Biol. 2002, 318, 503-18. DOI: https://doi.org/10.1016/S0022-2836(02)00051-7.
Weksberg, T.E.; Lynch, G.C.; Krause, K.L.; Pettitt, B.M. Biophys. J. 2007, 92, 3337-3345. DOI: https://doi.org/10.1529/biophysj.106.088096.

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2023 Rubén M. Carballo, Héctor A. Peniche-Pavía, Ramiro Quijano-Quiñones, David Cáceres-Castillo, Gumersindo Mirón-López, Manlio Graniel-Sabido, Andrea Reyes-Cuapio, Rosa E. Moo-Puc, Lía Valencia-Chan, Gonzalo Joaquín Mena-Rejón

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









