Impact of L-Pyroglutamic Acid on the Solubility of Puerarin: Preparation, Solid-State Characterization and Physicochemical Evaluation of Puerarin-L-Pyroglutamic Acid Co-Crystal
DOI:
https://doi.org/10.29356/jmcs.v67i2.1916Keywords:
Oral solubility, co-crystal, co-former, physicochemical properties, puerarin, solubility, dissolutionAbstract
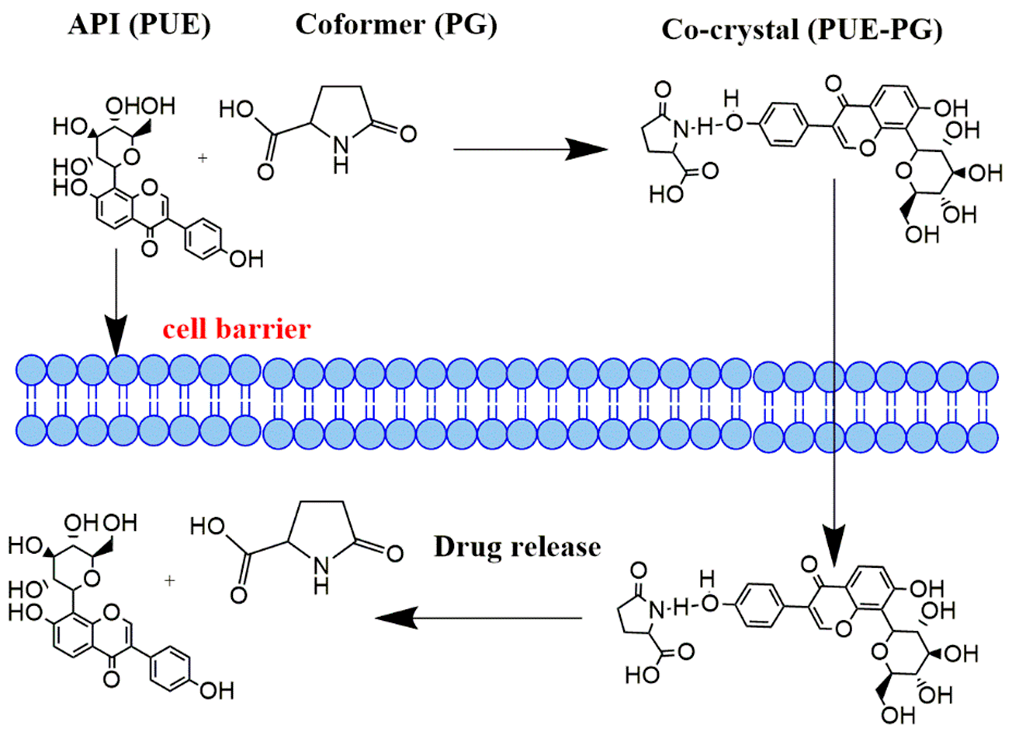
Abstract. Drug solubility plays a significant role in the successful therapeutic formulation. The objective of this work is enhancing the water solubility of Puerarin. We successfully synthesized a novel crystalline phase co-crystal of Puerarin (PUE) with L-pyroglutamic acid (PG) via recrystallization method and characterized by various solid-state characterization techniques. PXRD pattern shows the crystallinity phase co-crystal. The DSC analysis of co-crystal shows change in the thermal behavior compared with a pure form of PUE and PG. The FT-IR analysis shows change in the functional group frequency due to H-bonding interaction between PUE and PG molecule. The solubility of Pure PUE and co-crystal investigated in Pure water, pH 6.8 phosphate buffer solution and pH 1.2 acidic medium. co-crystal reveals improved solubility when compared with pure form of PUE. The time-dependent in vitro dissolution rate of co-crystal was more significant compared to the pure commercial form of PUE, demonstrating that co-crystal could be used as a useful product for pharmaceutical formulation with enhance properties.
Resumen. La solubilidad de un fármaco juega un papel importante en su formulación farmacútica final. El objetivo de este trabajo es incrementar la solubilidad acuosa del compuesto puerarina. En este sentido, reportamos la síntesis de una nueva matriz cristalina, formada a través de la recristalización de una mezcla de puerarina (PUE) y ácido L-piroglutámico (PG). El patron de análisis DSC del co-cristal mostró un cambio términco comparado con PUE y PG puros. El análisis detallado del co-cristal por medio de infrarrojo (FT-IR) mostró un cambio en la fecuancia de absorción en la región característica de enlaces de hidrógeno entre PUE y PG. Comparamos la solubilidad de una muestra pura de PUE y la de una muestra del co-cristal en agua, en un buffer de fosfátos pH 6.8, y en medio acídico a pH 1.2. La muestra del co-cristal mostró un aumento significativo en la solubilidad acuosa, comparada con la de PUE en todos los medios. Además, el perfil de disolución de una mestra del co-cristal fue significativamente mayor que el perfil de disolución de PUE, demostrando que esta forma de co-cristalización es un procedimiento altamente efectivo para incrementar la solubilidad acuosa de PUE.
Downloads
References
Duggirala, N.K.; Perry, M. L.; Almarsson, O.; Zaworotko, M. J. Chem. Commun. 2016, 52, 640–655. DOI: https://doi.org/10.1039/C5CC08216A
Chen, Y.; Li, L.; Yao, J.; Ma, Y. Y.; Chen, J. M.; Lu, T. B. Cryst. Growth. Des. 2016, 16, 2923–2930. DOI: https://doi.org/10.1021/acs.cgd.6b00266
Karimi-Jafari, M.; Padrela, L.; Walker, G. M.; Croker, D. Cryst. Growth. Des. 2018, 18, 6370–6387. DOI: https://doi.org/10.1021/acs.cgd.8b00933
Fael, H.; Barbas, R.; Prohens, R.; Ràfols, C.; Fuguet, E. Pharmaceutics. 2022, 14: 29. DOI: https://doi.org/10.3390/pharmaceutics14112310
Barbas, R.; Kumar, V.; Vallcorba, O.; Prohens, R.; Frontera, A. Crystals. 2020, 10, 1126. DOI: https://doi.org/10.3390/cryst10121126
Shan, N.; Perry, M. L.; Weyna, D. R.; Zaworotko, M. J. Drug. Metab. Toxicol. 2014, 10, 1255–1271. DOI: https://doi.org/10.1517/17425255.2014.942281
Nangia, A.; Desiraju, G.R. Acta Crystallogr. 1998, 54, 934-944. DOI: https://doi.org/10.1107/S0108767398008551
Aneta, M.; Szafert, S. J. Organometallic Chem. 2017, 847, 173–183. DOI: https://doi.org/10.1016/j.jorganchem.2017.05.044
Bo, Y.; Fang, J.; Zhang, Z.; Xue, J.; Liu, J.; Hong, Z.; Du, Y. Pharmaceutics. 2021, 13, 1303. DOI: https://doi.org/10.3390/pharmaceutics13081303
Zhou, Y.X.; Zhang, H.; Peng, C. Phytother. Res. 2014, 28, 961-975. DOI: https://doi.org/10.1002/ptr.5083
Kato, E.; Kawabata, J. Bioorg. Med. Chem. Lett. 2010, 20, 4333-4336. DOI: https://doi.org/10.1016/j.bmcl.2010.06.077
Yan, J.; Guan, Z.; Zhu, W.; Zhong, L.; Qiu, Z.Q.; Yue. P.; Wu, W.T.; Liu, J.; Huang, X. Pharmaceutics. 2020, 12, 216. DOI: https://doi.org/10.3390/pharmaceutics12030216
Li, H.; Dong, L.; Liu, Y.; Wang, G.; Wang, G.; Qiao, Y. Int. J. Pharm. 2014, 8, 466. DOI: https://doi.org/10.1016/j.ijpharm.2014.03.014
Inam, M.; Jiajia, W.; Jie S.; Phan, C.U. Tang, G.; Hu, X. Crystals. 2018, 8, 336. DOI: https://doi.org/10.3390/cryst8090336
Williams, H. D.; Trevaskis, N. L.; Charman, S. A.; Shanker, R. M.; Charman, W. N.; Pouton, C. W.; Porter, C. J. Pharmacol. Rev. 2013, 65, 315-499. DOI: https://doi.org/10.1124/pr.112.005660
Francisco, J. A.; Carolina, A.; Antonio, F.; Rafael, B.; Rafel, P.; Milena, D.; Alicia, D.; Jaime, G.; Duane, C. Pharmaceutics. 2021, 13, 2140.
Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Chem. Res. 2005, 38, 386-395. DOI: https://doi.org/10.1021/ar0400995
Liu, Y.; Lin, S. X.; Niu, R. J.; Liu, Q.; Zhang, W .H.; Young, D. J. Chem. Plus Chem. 2020, 85, 832-837. DOI: https://doi.org/10.1002/cplu.202000175
Newman, A.W.; Byrn, S. R. Drug. Disco Today. 2003, 8, 898-905. DOI: https://doi.org/10.1016/S1359-6446(03)02832-0
Sambas-evam, K. Int. J. Mol. Sci. 2003, 14, 3671.
Sugano, K.; Kataoka, M.; Mathews, C. C. S.; Yamashita, S. Eur. J. Pharm. Sci. 2010, 40, 118-124. DOI: https://doi.org/10.1016/j.ejps.2010.03.011
Ramesh, K.; Shekar, B.C.; Khadgapathi, P.; Bhikshapathi, D.; Renuka, K. Int. J. Drug. Deliv. 2015, 7, 32-43.
Inam, M.; Lu, L.; Wang. J.; Ka-Xi, Y.; Phan, C. U.; Jie, S.; Wen-Hua, Z.; Tang G.; Hu, X. Int. J. Mol. Sci. 2021, 22, 928. DOI: https://doi.org/10.3390/ijms22020928

Downloads
Published
Issue
Section
License
Copyright (c) 2023 Muhammad Inam, Muhammad Jamshed , Idrees Rehman, Wahib Noor Khan, Muhammad Iqbal Zaman, Muhammad Adnan Akram, Tayba Chudhary

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









