The Contribution of Dispersion to the Intrinsic Energy Barriers of Neutral Model Diels-Alder Reactions
DOI:
https://doi.org/10.29356/jmcs.v68i1.1867Keywords:
Diels-Alder reaction, Activation barrier, Intrinsic reaction coordinate, Dispersion interactions, Distortion/interaction modelAbstract
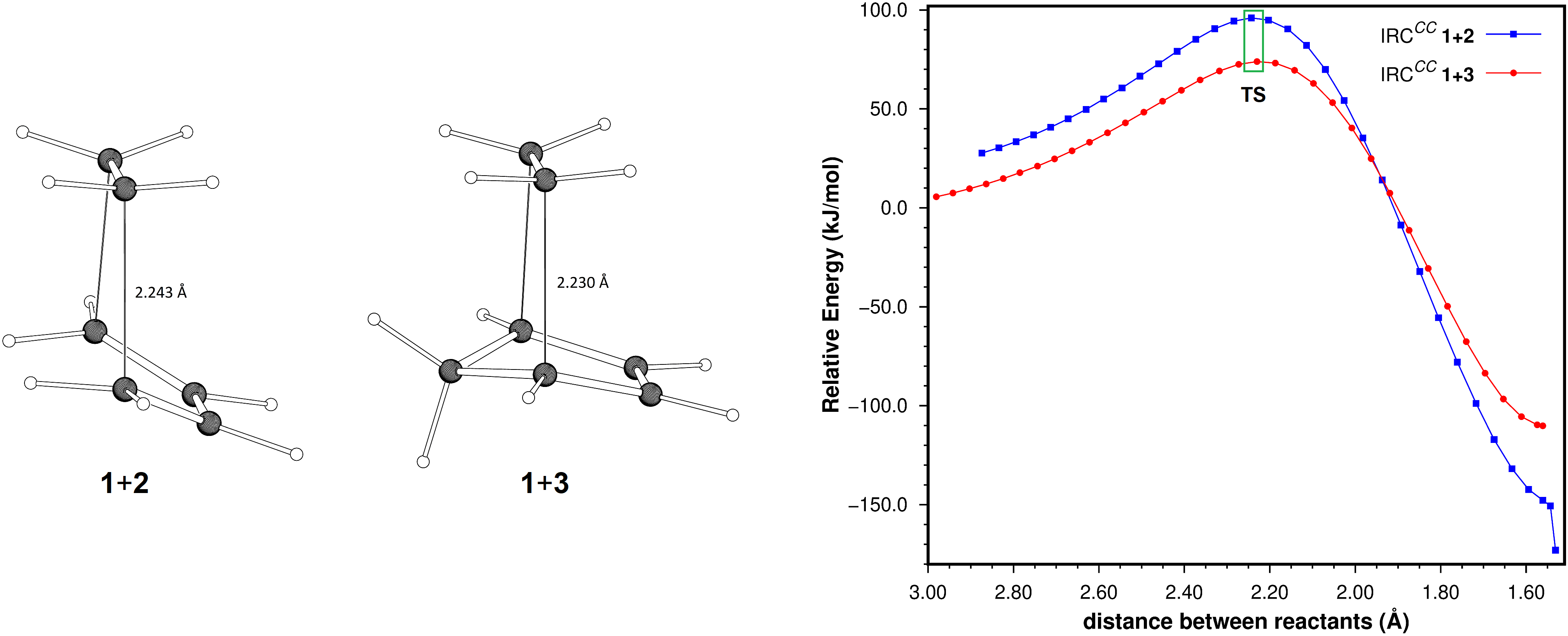
The intrinsic reaction coordinates for the cycloadditions between ethene and 1,3-butadiene, and ethene and cyclopentadiene, were determined at the SCS-MP2/aug-cc-pVTZ level of theory. The energy contents of the points determined for both coordinates were decomposed into their deformation and interaction contributions. From this analysis it is concluded that the higher reaction barrier for the butadiene-ethene cycloaddition can be attributed primarily to the conformational change of butadiene required by the reaction (higher deformation energy). There is also a minor contribution of the interaction term, which is more stabilizing for the cyclopentadiene-ethene reaction. An additional decomposition of these terms into their Hartree-Fock and SCS-MP2 correlation components suggests that the higher stabilization of the transition state of the cyclopentadiene-ethene cycloaddition is mostly due to stronger dispersion interactions between reactants, resulting from the larger contact surface between them, and not to stabilizing electronic effects.
Resumen. Se determinaron las coordenadas intrínsecas de reacción para las cicloadiciones entre eteno y 1,3-butadieno, y eteno y ciclopentadieno al nivel de teoría SCS-MP2/aug-cc-pVTZ. La energía de los puntos obtenidos en ambas coordenadas se descompuso en sus contribuciones de deformación e interacción. A partir de este análisis se concluye que la mayor barrera energética para la cicloadición eteno-butadieno puede atribuirse, principalmente, al cambio conformacional del butadieno requerido por la reacción (mayor energía de deformación). También se encuentra que el término de interacción es más estabilizante para la reacción entre ciclopentadieno y eteno, aunque la contribución de este término es menor. La descomposición adicional de las energías de interacción de estas reacciones en sus componentes de Hartree-Fock y de correlación SCS-MP2, sugiere que la mayor estabilización del estado de transición en la reacción entre ciclopentadieno y eteno, se debe principalmente a la interacción de dispersión más fuertemente estabilizante entre estos reactantes, resultado de la mayor superficie de contacto entre ellos y no a efectos electrónicos estabilizantes.
Downloads
References
Hoffmann, R.; Woodward, R. B. J. Am. Chem. Soc. 1965, 87, 4388-4389. DOI: https://doi.org/10.1021/ja00947a033.
Hoffmann, R.; Woodward, R. B. Acc. Chem. Res. 1968, 1, 17-22. DOI: https://doi.org/10.1021/ar50001a003.
Woodward, R. B.; Hoffmann, R., The Conservation of Orbital Symmetry. Academic Press: New York, 1970. DOI: https://doi.org/10.1016/B978-1-4832-3290-4.50006-4
Fukui, K.; Yonezawa, T.; Shingu, H. J. Chem. Phys. 1952, 20, 722-725. DOI: https://doi.org/10.1063/1.1700523.
Fukui, K. Acc. Chem. Res. 1971, 4, 57-64. DOI: https://doi.org/10.1021/ar50038a003.
Houk, K. N. Acc. Chem. Res. 1975, 8, 361-369. DOI: https://doi.org/10.1021/ar50095a001.
Fleming, I. Frontier Orbitals and Organic Chemical Reactions. John Wiley & Sons: Chichester, 1976.
Robiette, R.; Marchand-Brynaert, J.; Peeters, D. J. Org. Chem. 2002, 67, 6823-6826. DOI: https://doi.org/10.1021/jo025796u.
Domingo, L. R.; Aurell, M. J.; Pérez, P.; Contreras, R. Tetrahedron. 2002, 58, 4417-4423. DOI: https://doi.org/10.1016/S0040-4020(02)00410-6.
Domingo, L. R.; Saez, J. A. Org. Biomol. Chem. 2009, 7, 3576-3583. DOI: https://doi.org/10.1039/B909611F.
Domingo, L. R.; Chamorro, E.; Perez, P. Org. Biomol. Chem. 2010, 8, 5495-5504. DOI: https://doi.org/10.1039/C0OB00563K.
Sauer, J.; Lang, D.; Mielert, A. Angew. Chem., Int. Ed. 1962, 1, 268-269. DOI: https://doi.org/10.1002/anie.196202683.
Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry Part A: Structure and Mechanism. Fifth ed.; Springer: New York, 2007.
Bartlett, P. D.; Schueller, K. E. J. Am. Chem. Soc. 1968, 90, 6071-6077. DOI: https://doi.org/10.1021/ja01024a024.
Joshel, L. M.; Butz, L. W. J. Am. Chem. Soc. 1941, 63, 3350-3351. DOI: https://doi.org/10.1021/ja01857a033.
Walsh, R.; Wells, J. M. J. Chem. Soc., Perkin Trans. 2 1976, 52-55. DOI: https://doi.org/10.1039/P29760000052.
Rowley, D.; Steiner, H. Discuss. Faraday Soc. 1951, 10, 198-213. DOI: https://doi.org/10.1039/DF9511000198.
Smith, S. R.; Gordon, A. S. J. Phys. Chem. 1961, 65, 1124-1128. DOI: https://doi.org/10.1021/j100825a008.
Skinner, J. L.; Sliepcevich, C. M. Ind. Eng. Chem. Fundam. 1963, 2, 168-172. DOI: https://doi.org/10.1021/i160007a002.
Uchiyama, M.; Tomioka, T.; Amano, A. J. Phys. Chem. 1964, 68, 1878-1881. DOI: https://doi.org/10.1021/j100789a036.
Van Sickle, D. E.; Rodin, J. O. J. Am. Chem. Soc. 1964, 86, 3091-3094. DOI: https://doi.org/10.1021/ja01069a024.
Tardy, D. C.; Ireton, R.; Gordon, A. S. J. Am. Chem. Soc. 1979, 101, 1508-1514. DOI: https://doi.org/10.1021/ja00500a024.
Houk, K. N.; Lin, Y. T.; Brown, F. K. J. Am. Chem. Soc. 1986, 108, 554-556. DOI: https://doi.org/10.1021/ja00263a059.
Burke, L. A.; Leroy, G.; Sana, M. Theor. Chem. Acc. 1975, 40, 313-321. DOI: https://doi.org/10.1007/bf00668337.
Townshend, R. E.; Ramunni, G.; Segal, G.; Hehre, W. J.; Salem, L. J. Am. Chem. Soc. 1976, 98, 2190-2198. DOI: https://doi.org/10.1021/ja00424a031.
Burke, L. A.; Leroy, G. Theor. Chem. Acc. 1977, 44, 219-221. DOI: https://doi.org/10.1007/bf00549104.
Jug, K.; Krüger, H. W. Theor. Chem. Acc. 1979, 52, 19-26. DOI: https://doi.org/10.1007/bf00581697.
Bernardi, F.; Bottoni, A.; Robb, M. A.; Field, M. J.; Hillier, I. H.; Guest, M. F. J. Chem. Soc., Chem. Commun. 1985, 1051-1052. DOI: https://doi.org/10.1039/C39850001051.
Dewar, M. J. S.; Olivella, S.; Stewart, J. J. P. J. Am. Chem. Soc. 1986, 108, 5771-5779. DOI: https://doi.org/10.1021/ja00279a018.
Bernardi, F.; Bottoni, A.; Field, M. J.; Guest, M. F.; Hillier, I. H.; Robb, M. A.; Venturini, A. J. Am. Chem. Soc. 1988, 110, 3050-3055. DOI: https://doi.org/10.1021/ja00218a009.
Bach, R. D.; McDouall, J. J. W.; Schlegel, H. B.; Wolber, G. J. J. Org. Chem. 1989, 54, 2931-2935. DOI: https://doi.org/10.1021/jo00273a029.
Houk, K. N.; Loncharich, R. J.; Blake, J. F.; Jorgensen, W. L. J. Am. Chem. Soc. 1989, 111, 9172-9176. DOI: https://doi.org/10.1021/ja00208a006.
Jorgensen, W. L.; Lim, D.; Blake, J. F. J. Am. Chem. Soc. 1993, 115, 2936-2942. DOI: https://doi.org/10.1021/ja00060a048.
Li, Y.; Houk, K. N. J. Am. Chem. Soc. 1993, 115, 7478-7485. DOI: https://doi.org/10.1021/ja00069a055.
Herges, R.; Jiao, H.; Schleyer, P. v. R. Angew. Chem., Int. Ed. 1994, 33, 1376-1378. DOI: https://doi.org/10.1002/anie.199413761.
Houk, K. N.; Li, Y.; Storer, J.; Raimondi, L.; Beno, B. J. Chem. Soc., Faraday Trans. 1994, 90, 1599-1604. DOI: https://doi.org/10.1039/FT9949001599.
Bernardi, F.; Celani, P.; Olivucci, M.; Robb, M. A.; Suzzi-Valli, G. J. Am. Chem. Soc. 1995, 117, 10531-10536. DOI: https://doi.org/10.1021/ja00147a014.
Jursic, B.; Zdravkovski, Z. J. Chem. Soc., Perkin Trans. 2 1995, 1223-1226. DOI: https://doi.org/10.1039/P29950001223.
Goldstein, E.; Beno, B.; Houk, K. N. J. Am. Chem. Soc. 1996, 118, 6036-6043. DOI: https://doi.org/10.1021/ja9601494.
Torrent, M.; Durán, M.; Solà, M. SCIENTIA gerundensis. 1996, 22, 123-131.
Branchadell, V. Int. J. Quantum Chem. 1997, 61, 381-388. DOI: https://doi.org/10.1002/(sici)1097-461x(1997)61:3<381::aid-qua3>3.0.co;2-s. DOI: https://doi.org/10.1002/(SICI)1097-461X(1997)61:3<381::AID-QUA3>3.3.CO;2-8
Jiao, H.; Schleyer, P. v. R. J. Phys. Org. Chem. 1998, 11, 655-662. DOI: https://doi.org/10.1002/(SICI)1099-1395(199808/09)11:8/9<655::AID-POC66>3.0.CO;2-U. DOI: https://doi.org/10.1002/(SICI)1099-1395(199808/09)11:8/9<655::AID-POC66>3.3.CO;2-L
Spino, C.; Pesant, M.; Dory, Y. Angew. Chem., Int. Ed. 1998, 37, 3262-3265. DOI: https://doi.org/10.1002/(sici)1521-3773(19981217)37:23<3262::aid-anie3262>3.0.co;2-t.
Bradley, A. Z.; Kociolek, M. G.; Johnson, R. P. J. Org. Chem. 2000, 65, 7134-7138. DOI: https://doi.org/10.1021/jo000916o.
Sakai, S. J. Phys. Chem. A. 2000, 104, 922-927. DOI: https://doi.org/10.1021/jp9926894.
Huang, C.-H.; Tsai, L.-C.; Hu, W.-P. J. Phys. Chem. A. 2001, 105, 9945-9953. DOI: https://doi.org/10.1021/jp012740f.
Dinadayalane, T. C.; Vijaya, R.; Smitha, A.; Sastry, G. N. J. Phys. Chem. A. 2002, 106, 1627-1633. DOI: https://doi.org/10.1021/jp013910r.
Guner, V.; Khuong, K. S.; Leach, A. G.; Lee, P. S.; Bartberger, M. D.; Houk, K. N. J. Phys. Chem. A. 2003, 107, 11445-11459. DOI: https://doi.org/10.1021/jp035501w.
Lischka, H.; Ventura, E.; Dallos, M. ChemPhysChem 2004, 5, 1365-1371. DOI: https://doi.org/10.1002/cphc.200400104.
Sakai, S. J. Phys. Chem. A. 2006, 110, 6339-6344. DOI: https://doi.org/10.1021/jp0560011.
Hirao, H. J. Comput. Chem. 2008, 29, 1399-1407. DOI: https://doi.org/10.1002/jcc.20899.
Murray, J. S.; Yepes, D.; Jaque, P.; Politzer, P. Comput. Theor. Chem. 2015, 1053, 270-280. DOI: https://doi.org/10.1016/j.comptc.2014.08.010.
Scarborough, D. L. A.; Kobayashi, R.; Thompson, C. D.; Izgorodina, E. I. Int. J. Quantum Chem. 2015, 115, 989-1001. DOI: https://doi.org/10.1002/qua.24933.
Sexton, T.; Kraka, E.; Cremer, D. J. Phys. Chem. A 2016, 120, 1097-1111. DOI: https://doi.org/10.1021/acs.jpca.5b11493.
Domingo, L. R.; Ríos-Gutiérrez, M.; Pérez, P. Tetrahedron. 2017, 73, 1718-1724. DOI: https://doi.org/10.1016/j.tet.2017.02.012.
Chakraborty, D.; Das, R.; Chattaraj, P. K. ChemPhysChem. 2017, 18, 2162-2170. DOI: https://doi.org/10.1002/cphc.201700308.
Chen, B.; Hoffmann, R.; Cammi, R. Angew. Chem., Int. Ed. 2017, 56, 11126-11142. DOI: https://doi.org/10.1002/anie.201705427.
Casals-Sainz, J. L.; Francisco, E.; Martín Pendás, Á. Z. Anorg. Allg. Chem. 2020, 646, 1062-1072. DOI: https://doi.org/10.1002/zaac.202000038.
Jara-Cortés, J.; Leal-Sánchez, E.; Hernández-Trujillo, J. J. Phys. Chem. A. 2020, 124, 6370-6379. DOI: https://doi.org/10.1021/acs.jpca.0c04171.
Ayarde-Henríquez, L.; Guerra, C.; Duque-Noreña, M.; Rincón, E.; Pérez, P.; Chamorro, E. J. Phys. Chem. A. 2021, 125, 5152-5165. DOI: https://doi.org/10.1021/acs.jpca.1c01448.
McLachlan, A. D.; Ball, M. A. Rev. Mod. Phys. 1964, 36, 844-855. DOI: https://doi.org/10.1103/RevModPhys.36.844.
Jurečka, P.; Šponer, J.; Černý, J.; Hobza, P. Phys. Chem. Chem. Phys. 2006, 8, 1985-1993. DOI: https://doi.org/10.1039/b600027d.
Grimme, S. J. Chem. Phys. 2003, 118, 9095-9102. DOI: https://doi.org/10.1063/1.1569242.
Gerenkamp, M.; Grimme, S. Chem. Phys. Lett. 2004, 392, 229-235. DOI: https://doi.org/10.1016/j.cplett.2004.05.063.
Goumans, T. P. M.; Ehlers, A. W.; Lammertsma, K.; Würthwein, E.-U.; Grimme, S. Chem. - Eur. J. 2004, 10, 6468-6475. DOI: https://doi.org/10.1002/chem.200400250.
Piacenza, M.; Grimme, S. J. Comput. Chem. 2004, 25, 83-99. DOI: https://doi.org/10.1002/jcc.10365.
Grimme, S.; Mück-Lichtenfeld, C.; Würthwein, E.-U.; Ehlers, A. W.; Goumans, T. P. M.; Lammertsma, K. J. Phys. Chem. A 2006, 110, 2583-2586. DOI: https://doi.org/10.1021/jp057329x.
Hill, J. G.; Platts, J. A.; Werner, H.-J. Phys. Chem. Chem. Phys. 2006, 8, 4072-4078. DOI: https://doi.org/10.1039/B608623C.
Antony, J.; Grimme, S. J. Phys. Chem. A. 2007, 111, 4862-4868. DOI: https://doi.org/10.1021/jp070589p.
Takatani, T.; David Sherrill, C. Phys. Chem. Chem. Phys. 2007, 9, 6106-6114. DOI: https://doi.org/10.1039/B709669K.
Bates, D. M.; Anderson, J. A.; Oloyede, P.; Tschumper, G. S. Phys. Chem. Chem. Phys. 2008, 10, 2775-2779 DOI: https://doi.org/10.1039/B718720C.
Takatani, T.; Hohenstein, E. G.; Sherrill, C. D. J. Chem. Phys. 2008, 128, 124111-124117. DOI: https://doi.org/10.1063/1.2883974.
King, R. A. Mol. Phys. 2009, 107, 789-795. DOI: https://doi.org/10.1080/00268970802641242.
Riley, K. E.; Platts, J. A.; Řezáč, J.; Hobza, P.; Hill, J. G. J. Phys. Chem. A. 2012, 116, 4159-4169. DOI: https://doi.org/10.1021/jp211997b.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian, Inc.: Wallingford, CT, 2013.
Kesharwani, M. K.; Brauer, B.; Martin, J. M. L. J. Phys. Chem. A. 2015, 119, 1701-1714. DOI: https://doi.org/10.1021/jp508422u.
Boys, S. F.; Bernardi, F. Mol. Phys. 1970, 19, 553-566. DOI: https://doi.org/10.1080/00268977000101561.
NIST Chemistry Webbook. http://webbook.nist.gov, accessed in June 2022.
Alvarez-Idaboy, J. R.; Galano, A. Theor. Chem. Acc. 2010, 126, 75-85. DOI: https://doi.org/10.1007/s00214-009-0676-z.
Xidos, J. D.; Poirier, R. A.; Pye, C. C.; Burnell, D. J. J. Org. Chem. 1998, 63, 105-112. DOI: https://doi.org/10.1021/jo9712815.
Houk, K. N.; Gandour, R. W.; Strozier, R. W.; Rondan, N. G.; Paquette, L. A. J. Am. Chem. Soc. 1979, 101, 6797-6802. DOI: https://doi.org/10.1021/ja00517a001.
Coxon, J. M.; Grice, S. T.; Maclagan, R. G. A. R.; McDonald, D. Q. J. Org. Chem. 1990, 55, 3804-3807. DOI: https://doi.org/10.1021/jo00299a021.
Ess, D. H.; Houk, K. N. J. Am. Chem. Soc. 2007, 129, 10646-10647. DOI: https://doi.org/10.1021/ja0734086.
Ess, D. H.; Houk, K. N. J. Am. Chem. Soc. 2008, 130, 10187-10198. DOI: https://doi.org/10.1021/ja800009z.
Jones, G. O.; Houk, K. N. J. Org. Chem. 2008, 73, 1333-1342. DOI: https://doi.org/10.1021/jo702295d.
Hayden, A. E.; Houk, K. N. J. Am. Chem. Soc. 2009, 131, 4084-4089. DOI: https://doi.org/10.1021/ja809142x.
Bickelhaupt, F. M.; Houk, K. N. Angew. Chem., Int. Ed. 2017, 56, 10070-10086. DOI: https://doi.org/10.1002/anie.201701486.
Bickelhaupt, F. M. J. Comput. Chem. 1999, 20, 114-128. DOI: https://doi.org/10.1002/(SICI)1096-987X(19990115)20:1<114::AID-JCC12>3.0.CO;2-L.
Diefenbach, A.; Bickelhaupt, F. M. J. Phys. Chem. A 2004, 108, 8460-8466. DOI: 10.1021/jp047986+. DOI: https://doi.org/10.1021/jp047986+
De Jong, G. T.; Bickelhaupt, F. M. ChemPhysChem 2007, 8, 1170-1181. DOI: https://doi.org/10.1002/cphc.200700092.
Van Zeist, W.-J.; Bickelhaupt, F. M. Org. Biomol. Chem. 2010, 8, 3118-3127. DOI: https://doi.org/10.1039/B926828F.
Fernández, I.; Bickelhaupt, F. M. Chem. Soc. Rev. 2014, 43, 4953-4967. DOI: https://doi.org/10.1039/C4CS00055B.
Wolters, L. P.; Bickelhaupt, F. M. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2015, 5, 324-343. DOI: https://doi.org/10.1002/wcms.1221.
Eisler, B.; Wassermann, A. Discuss. Faraday Soc. 1951, 235. DOI: https://doi.org/10.1039/DF9511000213.
Eisler, B.; Wassermann, A. J. Chem. Soc. 1953, 979-982. DOI: https://doi.org/10.1039/JR9530000979.
Craig, D.; Shipman, J. J.; Fowler, R. B. J. Am. Chem. Soc. 1961, 83, 2885-2891. DOI: https://doi.org/10.1021/ja01474a023.
Morokuma, K. J. Chem. Phys. 1971, 55, 1236-1244. DOI: https://doi.org/10.1063/1.1676210.
Kitaura, K.; Morokuma, K. Int. J. Quantum Chem. 1976, 10, 325-340. DOI: https://doi.org/10.1002/qua.560100211.

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2023 Dr. Hugo Alejandro Jiménez Vázquez, M. en C. Luis Almazán Sánchez, Dra. Adriana Benavides Macias

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









