Improved Knoevenagel Condensation Protocol for the Synthesis of Cyanoacrylates and their Anticancer Activity
DOI:
https://doi.org/10.29356/jmcs.v67i1.1835Keywords:
ethyl cyanoacetate, Diisopropyl ethyl ammonium acetate(DIPEAc), cyanoacrylate, knoevenagel condensation, anticancer activityAbstract
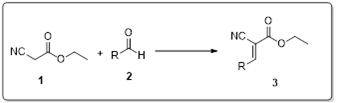
DIPEAc (diisopropylethylammonium acetate) has been used as a catalyst in the Knoevenagel condensation of aldehydes and ethyl cyanoacetate to produce cyanoacrylate with high yields. The reaction's key characteristics are a shorter reaction time, a large substrate scope, the viability of different functional groups, ease of work-up, and high yields, which provide environmental benefits. Furthermore, the anticancer activity of the synthesized series of cyanoacrylate derivatives was tested against A549, HT-29, and HepG2 cell lines.
Resumen. El aetato de diisopropiletilamonio (DIPEAc) se empleó como catalizador en la reacción de condensación de Knoevenagel emplenado aldehídos y cianoacetato de etilo para producir cianoacrilatos en reindimientos elevados. Algunas características de esta aplicación es el que requiere tiempos cortos de reacción, se puede practicar en una amplia gama de sustratos, tolera varios grupos funcionales, el trabajo de la reacción es sencillo y tiene elvados rendimientos, lo que es compatible con el medio ambiente. Los productos obtenidos fueron probados con las líneas celulares A549, HT-29 y HepG2.
Downloads
References
Lombardo, M.; Pasi, F.; Easwar, S.; Trombini, C. Adv. Synth. Catal. 2007, 349, 2061–2065. DOI: https://doi.org/10.1002/adsc.200700136
Zhou, L.; Wang, L. Chem. Lett. 2007,36, 628-629. DOI: https://doi.org/10.1246/cl.2007.628
Siyutkin D.E.; Kucherenko A.S.; Struchkova M.I.; Zlotin S.G. Tetrahedron Lett. 2008, 49, 1212-1216. DOI: https://doi.org/10.1016/j.tetlet.2007.12.044
Ni, B.; Headley, A.D. Chem. Eur. J. 2010, 16, 4426-4436. DOI: https://doi.org/10.1002/chem.200902747
. Handy, S.T. J. Org. Chem. 2006, 71, 4659-4662. DOI: https://doi.org/10.1021/jo060536o
Anjaiah, S.; Chandrasekhar, S.; Gree, R. J. Mol. Catal. A: Chem.. 2004, 214, 103-106.
Vijaya Durga, T.; Rambabu, A.; Srinivas Reddy, M.; Hari Babu, B. Asian. J. Chem. 2017, 29, 1313-1316. DOI: https://doi.org/10.14233/ajchem.2017.20479
Sowjanya, P.; Srinivasa Rao, V.; Srinivas Reddy, M.; Md Nayeem, S.K.; Haribabu, B. J. Chem. Thermodyn.. 2021, 154, 106330.
Knoevenagel, E. Ber. Dtsch. Chem. Ges. 1894, 27, 2345-2346. DOI: https://doi.org/10.1002/cber.189402702229
Rodionow, W. M. J. Am. Chem. Soc. 1929, 51, 847-852. DOI: https://doi.org/10.1021/ja01378a028
Wang, Y; Shang, Z.; Wu, T.; Fan, J.; Chen, J.; J. Mol. Catal. A: Chem. 2006, 253, 212-221. DOI: https://doi.org/10.1016/j.molcata.2006.03.035
Zhu, L.; Lei, N.; Miao, Z.; Sheng, C.; Zhuang, C.; Yao, J.; Zhang, W. Chin. J. Chem. 2012, 30, 139-143. DOI: https://doi.org/10.1002/cjoc.201180455
Gupta, M.; Wakhloo, B.P. ARKIVOC, 2007, 94-98. DOI: https://doi.org/10.3998/ark.5550190.0008.110
Sanjoy, K.;. Nimalini, M; Warjeet, S.L. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2015, 54B, 1157-1161.
Rajesh Krihnan, G.P.; Sreekumar, K. Tetrahedron Lett. 2014, 55, 2352-2354. DOI: https://doi.org/10.1016/j.tetlet.2014.02.084
Zhang, Q.; Ma, X.M.; Wei, H.X.; Zhao, X.; Luo, J. RSC Adv. 2017, 7, 53861-53870. DOI: https://doi.org/10.1039/C7RA10692K
Wan, J.P.; Jing, Y.; Liu, Y.; Sheng, S.; RSC Adv. 2014, 4, 63997-64000. DOI: https://doi.org/10.1039/C4RA13826K
de Paula, B.R.S.; Zampieri, D.S.; Zukerman-Schpector, J.; Tiekink, E.R.T.;. Rodrigues, J.A.R;. Moran, P. J.S. J. Braz. Chem. Soc. 2012, 23 (5), 825-830. DOI: https://doi.org/10.1590/S0103-50532012000500006
Kolahdoozan, M.; Kalbasi, R.J.; Shahzeidi, Z.S.; Zamani, F. Journal of Chemistry. 2013, https://doi.org/10.1155/2013/496837.
Beurden, K.V; de Koning, S.; Molendijk, D.; Schijndel, J.V. Green Chem. Lett. Rev. 2020, 13, 349-364. DOI: https://doi.org/10.1080/17518253.2020.1851398
Ashok, S.P.; Aravind, S.B.; Devkate, S.S. Asian. J. Chem. 2020, 32, 575-579. DOI: https://doi.org/10.14233/ajchem.2020.22432
Ruger, N.; Fassauer, G.M.; Bock, C.; Emmrich, T.; Bodtke, A.; Link, A. Mol. Diversity. 2017, 21(1), 9-27. DOI: https://doi.org/10.1007/s11030-016-9711-x
Naveen, S.; Lakshmi, S.; Dinesh, M.; Alpesh, P.; Anamik, S.; Sridhar, M.A.; Prasad, J.S. J. Chem. Crystallogr. 2007, 37, 733-738. DOI: https://doi.org/10.1007/s10870-007-9241-6
Guchhait, S.K.; Sisodiya, S.;. Saini, M; Shah, Y.V.;. Kumar, G; Daniel, D.P.; Hura, N.; Chaudhary, V. J. Org. Chem. 2018, 83, 5807-5815. DOI: https://doi.org/10.1021/acs.joc.8b00465
(a)Porter, D.W.; Bardley, M.; Brown, Z.; Charlton, S.J.; Cox, B.; Hunt, P.; Janus, D.; Lewis, S.; Oakey, P.; Connor, D.O.; Reilly, J.; Smith, N.; Press, N. J. Bioorg. Med. Chem. Lett. 2014, 24, 3285-3290. (b)Youseff, M.; Mohamed, H.M.;. Czezowski, C; Ata, A.; Abd-El-Aziz, A.S. Heterocycles. 2006, 68(2), 347-355. DOI: https://doi.org/10.3987/COM-05-10609
Surendarnathareddy, O.; Surya Narayana, Ch.V.; . Sharmila, N; Ramana, G.V.; Anuradha, V.; Hari Babu, B. Lett. Drug Des. Discovery. 2013, 10, 699-705. DOI: https://doi.org/10.2174/15701808113109990007
Ramesh, N.; Gangadhara Rao, M.; Tirumala, M.; Uma, M.V.; Hari Babu, B. Asian. J. Chem., 2016, 28, 1321-1324.
Jadhav, C.K.; Nipate, A.S.; Chate, A.V.; Songire, V.D.;. Patil, A.P; Gill, C.H. ACS Omega, 2019, 4, 22313-22324. DOI: https://doi.org/10.1021/acsomega.9b02286
Jadhav, C.K.; Nipate, A.S.; Chate, A.V.; Dofe, V.S.; Sangishetti, J.N.; Khedkar, V.M.; Gill, C.H. ACS Omega. 2020, 45, 29055-29067. DOI: https://doi.org/10.1021/acsomega.0c03575
Surendarnathareddy, O.; Baby Ramana, M.; Vijaya Durga, T.; Basavaiah, C.; Rajya Lakshmi, C.; Mokesh, R.G.; Vijaya, K.; Hari Babu, B. ChemistrySelect. 2020, 5, 8194-8197. DOI: https://doi.org/10.1002/slct.202001668

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2022 HARI BABU BOLLIKOLLA, santhi, krishna

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









