Synthesis of Novel Benzylic 1,2,3-triazole-4-carboxamides and their in vitro Activity Against Clinically Common Fungal Species
DOI:
https://doi.org/10.29356/jmcs.v65i2.1457Keywords:
1,2,3-Triazoles, antifungal activity, 1,3-dipolar cycloaddition, Rhizopus oryzaeAbstract
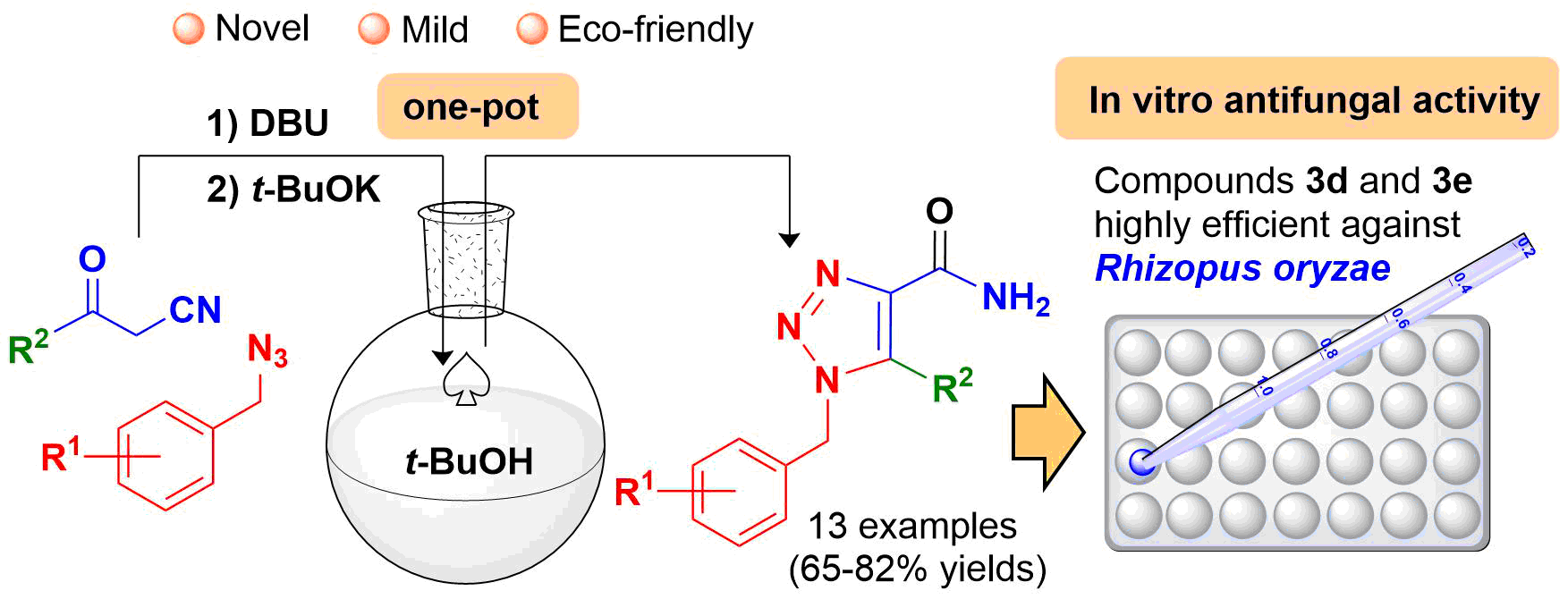
Abstract. A library of novel benzylic 1,2,3-triazole-4-carboxamides (3a-m) were obtained with acceptable yields via a one-pot procedure. The series of compounds was screened for fungicidal activity and evaluated in vitro against four filamentous fungi and four Candida species. The former consisted of Aspergillus fumigatus, Trichosporon cutaneum, Rhizopus oryzae and Mucor hiemalis, and the latter C. krusei, C. albicans, C. utilis and C. glabrata. According to the in vitro assays, 3d and 3e were the most efficient fungicidal agents (of all the test compounds) against R. oryzae, even better than the reference drug (itraconazole). Thus, 3d and 3e represent important scaffolds that can be modified to increase antifungal activity. Additionally, they are candidates for complementary studies on the inhibition of clinical infections produced by Rhizopus spp. strains.
Resumen. Se obtuvo una librería de nuevos bencil 1,2,3-triazoles-4-carboxamidas (3a-m) con rendimientos aceptables mediante un procedimiento one-pot. La serie de compuestos se seleccionó para determinar la actividad fungicida llevando a cabo una evaluación in vitro contra cuatro hongos filamentosos y cuatro especies de Candida. Los primeros consistieron en Aspergillus fumigatus, Trichosporon cutaneum, Rhizopus oryzae y Mucor hiemalis, mientras que para las segundas especies, esta fueron C. krusei, C. albicans, C. utilis y C. glabrata. Según los ensayos in vitro, 3d y 3e fueron los agentes fungicidas más eficaces (de todos los compuestos de prueba) contra R. oryzae, incluso mejores que el fármaco de referencia (itraconazol). Por tanto, 3d y 3e representan importantes núcleos que podrían modificarse para aumentar la actividad antifúngica, siendo excelentes candidatos para estudios complementarios sobre la inhibición de infecciones clínicas producidas por Rhizopus spp.
Downloads
References
Rodrigues, M. L.; Nosanchuk, J. D. PLoS Negl. Trop. Dis. 2020, 14, e0007964. https://doi.org/10.1371/journal.pntd.0007964
Köhler, J. R.; Casadevall, A.; Perfect, J. Cold Spring Harb. Perspect. Med. 2015, 5, a019273. https://doi.org/10.1101/cshperspect.a019273
Köhler, J. R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J. R., Chapter 39: Fungi that Infect Humans. In: The Fungal Kingdom. Heitman, J.; Howlett, B. J.; Crous, P. W.; Stukenbrock, E. H.; James, T. Y.; Gow, N. A. R. (Ed)., American Society for Microbiology 2017, e-ISBN: 9781555819583. https://doi.org/10.1128/microbiolspec.FUNK-0014-2016
Dheer, D.; Singh, V.; Shankar, R. Bioorg. Chem. 2017, 71, 30-54. https://doi.org/10.1016/j.bioorg.2017.01.010
Kharb, R.; Sharma, P. C.; Yar, M. S. J. Enzym. Inhib. Med. Chem. 2011, 26, 1–21. https://doi.org/10.3109/14756360903524304
de Carvalho da Silva, F.; Cardoso, M. F. C.; Ferreira, P. G.; Ferreira V. F. Biological Properties of 1H-1,2,3- and 2H-1,2,3-Triazoles. In: Dehaen W., Bakulev V. (eds) Chemistry of 1,2,3-triazoles. Topics in Heterocyclic Chemistry, vol 40, Springer, Cham, 2014. Online ISBN 978-3-319-07962-2. https://doi.org/10.1007/7081_2014_124
Massarotti, A.; Aprile, S.; Mercalli, V.; Del Grosso, E.; Grosa, G.; Sorba, G.; Tron, G. C. Chem. Med. Chem. 2014, 9, 2497–2508. https://doi.org/10.1002/cmdc.201402233
Agalave, S. G.; Maujan, S. R.; Pore, V. S., Chem. Asian J. 2011, 6, 2696–2718. https://doi.org/10.1002/asia.201100432
Howard, K. C.; Dennis, E. K.; Watt, D. S.; Garneau-Tsodikova, S. A. Chem. Soc. Rev. 2020, 49, 2426-2480. https://doi.org/10.1039/c9cs00556k
Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. RSC Adv. 2020, 10, 5610-5635. https://doi.org/10.1039/C9RA09510A
Lass-Flörl, C. Drugs 2011, 71, 2405–2419. https://doi.org/10.2165/11596540-000000000-00000
Nett, J. E.; Andes, D. R. Infect. Dis. Clin. North Am. 2016, 30, 51–83. https://doi.org/10.1016/j.idc.2015.10.012
Miceli, M. H.; Kauffman, C. A. Clin. Infect. Dis. 2015, 61, 1558–1565. https://doi.org/10.1093/cid/civ571
Chang, Y. L.; Yu, S. J.; Heitman, J.; Wellington, M.; Chen, Y. L. Virulence 2017, 8, 222–236. https://doi.org/10.1080/21505594.2016.1257457
Seyedmousavi, S.; Verweij, P. E.; Mouton, J. W. Expert Rev. Anti. Infect. Ther. 2015, 13, 9–27. https://doi.org/10.1586/14787210.2015.990382
Peyton, L. R.; Gallagher, S.; Hashemzadeh, M. Drugs Today (Barc) 2015, 51, 705–718. https://doi.org/10.1358/dot.2015.51.12.2421058
Chitasombat, M. N.; Kontoyiannis, D. P. Expert Opin. Pharmacother. 2015, 16, 1543–1558. https://doi.org/10.1517/14656566.2015.1057500
Revie, N. M.; Iyer, K. R.; Robbins, N.; Cowen, L. E. Curr. Opin. Microbiol. 2018, 45, 70–76. https://doi.org/10.1016/j.mib.2018.02.005
Perlin, D. S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. Lancet Infect. Dis. 2017, 17, 383–392. https://doi.org/10.1016/S1473-3099(17)30316-X
Beardsley, J.; Halliday, C. L.; Chen, S.; Sorrell, T. C. Future Microbiol. 2018, 13, 1175–1191. https://doi.org/10.2217/fmb-2018-0059
Lopez-Ribot, J. L.; Wiederhold, N. P.; Patterson T. F., Fungal Drug Resistance: Azoles. In: Mayers, D.; Sobel, J.; Ouellette, M.; Kaye, K.; Marchaim, D. (eds), Antimicrobial Drug Resistance. Springer, Cham, 2017, Online ISBN 978-3-319-46718-4, https://doi.org/10.1007/978-3-319-46718-4_27
Caramalho, R.; Tyndall, J. D. A.; Monk, B. C. Sci Rep. 2017, 7, 15898. https://doi.org/10.1038/s41598-017-16123-9
Dannaoui, E. Int. J. Antimicrob. Agents 2017, 50, 617-621. https://doi.org/10.1016/j.ijantimicag.2017.08.010
Odds, F. C.; Brown, A. J. P.; Gow, N. A. R. Trends Microbiol. 2003, 11, 272-279. https://doi.org/10.1016/s0966-842x(03)00117-3
Balding, P. R.; Porro, C. S., Munro, A. W.; Visser, S. P. J. Phys. Chem. A 2008, 112, 12911–12918. https://doi.org/10.1021/jp802087w
Zhang, Y.; Damu, G. L. V.; Cui, S. F.; Mi, J. L.; Tangadanchu, V. K. R.; Zhou, C. H. Med. Chem. Commun., 2017, 8, 1631-1639. https://doi.org/10.1039/C7MD00112F
Mast, N.; Zheng, W.; Stout, C. D.; Pikuleva, I. A. Molecular Pharmacology 2013, 84, 86-94; https://doi.org/10.1124/mol.113.085902
Kaushik, C. P.; Luxmi, R.; Kumar, M.; Singh, D.; Kumar, K.; Pahwa, A. Synth. Commun. 2019, 49, 118-128. https://doi.org/10.1080/00397911.2018.1544371
Thanh, N. D.; Hai, D. S.; Bich, V. T. N.; Hien, P. T. T.; Duyen, N. T. K.; Mai, N. T.; Dung, T. T.; Toan, V. N.; Van, H. T. K.; Dang, L. H.; Toan, D. N.; Van, T. T. T. Eur. J. Med. Chem. 2019, 167, 454-471. https://doi.org/10.1016/j.ejmech.2019.01.060
Fu, N.; Wang, S.; Zhang, Y.; Zhang, C.; Yang, D.; Weng, L.; Zhao, B.; Wang, L. Eur. J. Med. Chem. 2017, 136, 596-602, https://doi.org/10.1016/j.ejmech.2017.05.001
Aneja, B.; Irfan, M.; Kapil, C.; Jairajpuri, M. A.; Maguire, R.; Kavanagh, K.; Rizvi, M. M. A.; Manzoor, N.; Azam, A.; Abid, M. Org. Biomol. Chem. 2016, 14, 10599-10619. https://doi.org/10.1039/C6OB01718E
Dai, Z. H.; Chen, Y. F.; Zhang, M.; Li, S. K.; Yang, T. T.; Shen, L.; Wang, J. X.; Qian, S. S.; Zhu, H. L.; Ye, Y. H. Org. Biomol. Chem. 2015, 13, 477-486. https://doi.org/10.1039/C4OB01758G
Ramírez-Villalva, A.; González-Calderón, D.; Rojas-García, R. I.; González-Romero, C.; Tamaríz-Mascarúa, J.; Morales-Rodríguez, M.; Zavala-Segovia, N.; Fuentes-Benítes, A. Med. Chem. Commun. 2017, 8, 2258–2262, https://doi.org/10.1039/c7md00442g
González-Calderón, D.; Mejía-Dionicio, M. G.; Morales-Reza, M. A.; Ramírez-Villalva, A.; Morales-Rodríguez, M.; Jauregui-Rodríguez, B.; Díaz-Torres, E.; González-Romero, C.; Fuentes-Benítes, A. Eur. J. Med. Chem. 2016, 112, 60-65. https://doi.org/10.1016/j.ejmech.2016.02.013
González-Calderón, D.; Mejía-Dionicio, M. G.; Morales-Reza, M. A.; Aguirre-de Paz, J. G.; Ramírez-Villalva, A.; Morales-Rodríguez, M.; Fuentes-Benítes, A.; González-Romero, C. Bioorg. Chem. 2016, 69, 1–6, https://doi.org/10.1016/j.bioorg.2016.09.003
Ballari, M. S.; Herrera-Cano, N.; Lopez, A. G.; Wunderlin, D. A.; Feresín, G. E.; Santiago, A. N. J. Agric. Food Chem. 2017, 65, 10325–10331. https://doi.org/10.1021/acs.jafc.7b04130
Brand, S.; Ko, E. J.; Viayna, E.; Thompson, S.; Spinks, D.; Thomas, M.; Sandberg, L.; Marco, M.; Miles, T. J.; Read, K. D.; Gilbert, I. H. J. Med. Chem. 2017, 60, 7284–7299. https://doi.org/10.1021/acs.jmedchem.7b00463
Shaikh, M. H.; Subhedar, D. D.; Nawale, L.; Sarkar, D.; Khan, F. A. K.; Sangshetti, J. N.; Shingate, B. B. Med. Chem. Commun. 2015, 6, 1104-1116. https://doi.org/10.1039/C5MD00057B
Kamal, A.; Rao, A. V. S.; Vishnuvardhan, M. V. P. S.; Reddy, T. S.; Swapna, K.; Bagul, C.; Reddy, N. V. S.; Srinivasulu, V. Org. Biomol. Chem. 2015, 13, 4879-4895. https://doi.org/10.1039/C5OB00232J
Irfan, M.; Alam, S.; Manzoor, N.; Abid, M. PLoS ONE 2017, 12, e0175710. https://doi.org/10.1371/journal.pone.0175710
Shaikh, M. H.; Subhedar, D. D.; Khan, F. A. K.; Sangshetti, J. N.; Shingate, B. B. Chin. Chem. Lett. 2016, 27, 295-301. https://doi.org/10.1016/j.cclet.2015.11.003
National Committee for Clinical Laboratory Standards Institute (CLSI), Document M38-A2: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, Approved Standard, Second Edition, Clinical and Laboratory Standards Institute, Wayne, PA, 2002.
Barry, A. L. An overview of the Clinical and Laboratory Standards Institute (CLSI) and its impact on antimicrobial susceptibility tests, in: Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. (Eds.), Antimicrobial Susceptibility Testing Protocols, CRC Press Taylor & Francis Group, Florida, 2007, 1-6. ISBN: 9780824741006. DOI: https://doi.org/10.1201/9781420014495.ch1
Espinel-Ingroff, A.; Canton, E. Antifungal susceptibility testing of filamentous fungi, in: Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. (Eds.), Antimicrobial Susceptibility Testing Protocols, CRC Press Taylor & Francis Group, Florida, 2007, 209-241. ISBN: 9780824741006. DOI: https://doi.org/10.1201/9781420014495.ch10
National Committee for Clinical and Laboratory Standards Institute (CLSI), M27-A3: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard, Third Edition, Clinical and Laboratory Standards Institute, Wayne, PA, 2008. ISBN: 1-56238-666-2.
Espinel-Ingroff, A.; Cantón, E. Antifungal susceptibility testing of yeasts, in: Schwalbe, R.; Steele-Moore, L.; Goodwin, A. C. (Eds.), Antimicrobial Susceptibility Testing Protocols, CRC Press Taylor & Francis Group, Florida, 2007, 173-208. ISBN: 9780824741006. DOI: https://doi.org/10.1201/9781420014495.ch9
Fothergill, A. W. Antifungal Susceptibility Testing: Clinical Laboratory and Standards Institute (CLSI) methods, in: G.S. Hall (Ed.), Interactions of Yeasts, Moulds, and Antifungal Agents. How to Detect Resistance, Springer Science-Business Media, 2012, 65-74. https://doi.org/10.1007/978-1-59745-134-5_2
Vigezzi, C.; Riera, F. O.; Caeiro, J. P.; Sotomayor, C. E. Rev. Argent. Microbiol. 2020, In Press, https://doi.org/10.1016/j.ram.2020.06.003
Choi, S.; Song, J. S.; Woo, J. H.; Kim, S. H. Mycoses 2019, 62, 1006-14. https://doi.org/10.1111/myc.12994
Aguirre-De Paz, J. G.; González-Calderón, D.; Fuentes-Benítes, A.; González-Romero, C. Tet Lett. 2018, 59, 1760–1762. https://doi.org/10.1016/j.tetlet.2018.03.075
Vargas-Herrera, N.; Saavedra-Velasco, M.; Pichardo-Rodriguez, R. Acta Méd. Peru. 2020, 36, 287-290. DOI: https://doi.org/10.35663/amp.2019.364.903
Valdés, T. G. E.; Martínez, B. M. E.; Morayta, R. C. A. R. R. Rev. Latin. Infect. Pediatr. 2020, 33, 49-56. https://doi.org/10.35366/92386

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









