In vitro Activity of Picroside I in Type 2 Diabetes Based on Oxidative Stress
DOI:
https://doi.org/10.29356/jmcs.v67i2.1899Keywords:
Picroside I, oxidative stress, insulin resistance (IR), glucose consumption, HepG2 cellsAbstract
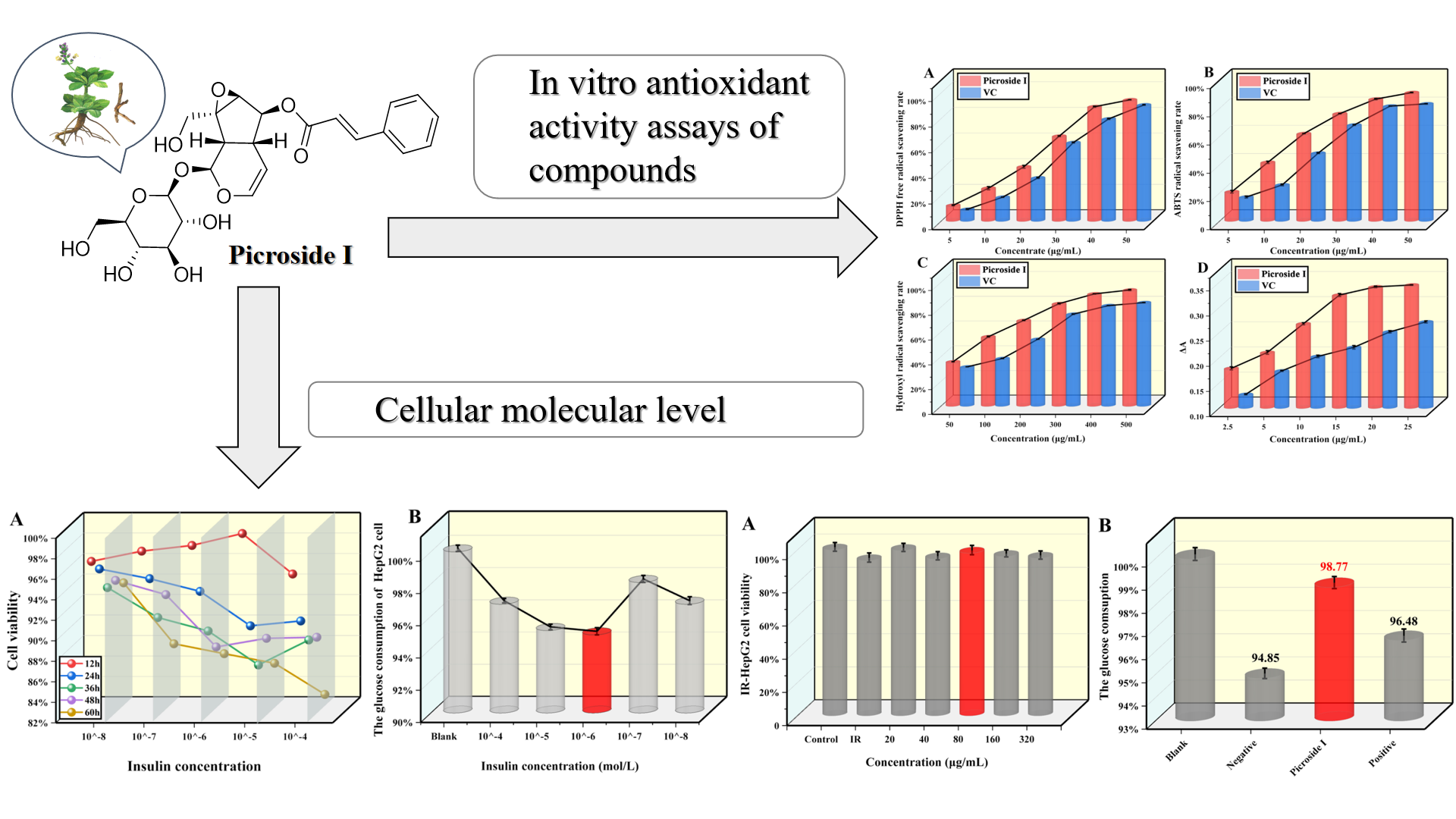
Abstract. The primary factor leading to insulin resistance (IR) and type 2 diabetes mellitus (T2DM) is oxidative stress. Despite its liver-protecting, enzyme-lowering, immune-regulating, and antiviral effects, the impact of picroside I on oxidative stress, glucose utilization, and IR has not been investigated yet. In vitro studies were conducted to evaluate the antioxidant properties of different concentrations of picroside I. The results showed that picroside I effectively suppresses α-glucosidase and α-amylase with IC50 values of 109.75 μg/mL and 160.71 μg/mL in the range of 50-500 μg/mL. Additionally, when IR-HepG2 cells were treated with 80 μg/mL of picroside I, it was found to have little effect on cell viability, increase glucose consumption, decrease the levels of the free radical metabolite malonic dialdehyde, and increase superoxide dismutase activity. These findings indicate that picroside I has the potential to regulate oxidative stress in IR-HepG2 cells, potentially improving IR and exhibiting anti-T2DM activity.
Resumen. El factor principal que conduce a la resistencia a la insulina (IR) y a la diabetes mellitus tipo 2 (T2DM) es el estrés oxidativo. A pesar de sus efectos protectores del hígado, reductores de enzimas, inmunorreguladores y antivirales, aún no se ha investigado el impacto del picrósido I sobre el estrés oxidativo, la utilización de glucosa y la IR. Se realizaron estudios in vitro para evaluar las propiedades antioxidantes de diferentes concentraciones de picrósido I. Los resultados mostraron que el picrósido I suprime eficazmente la α-glucosidasa y la α-amilasa con valores IC50 de 109,75 μg/mL y 160,71 μg/mL en el rango de 50 -500 microgramos/ml. Además, cuando las células IR-HepG2 se trataron con 80 μg/mL de picrósido I, se encontró que tenía poco efecto sobre la viabilidad celular, aumentaba el consumo de glucosa, disminuía los niveles del metabolito de radicales libres dialdehído malónico y aumentaba la actividad de la superóxido dismutasa. Estos hallazgos indican que el picrósido I tiene el potencial de regular el estrés oxidativo en las células IR-HepG2, mejorando potencialmente la IR y exhibiendo actividad anti-T2DM.
Downloads
References
Biessels, G. J.; Gispen, W. H. Neurobiol. Aging. 2005, 26 Suppl 1, 36-41. DOI: http://dx.doi.org/10.1016/j.neurobiolaging.2005.08.015 DOI: https://doi.org/10.1016/j.neurobiolaging.2005.08.015
Whiting, D. R.; Guariguata, L.; Weil, C.; Shaw, J. Diabetes Res. Clin. Prac. 2011, 94, 311-321 DOI: http://dx.doi.org/10.1016/j.diabres.2011.10.029 DOI: https://doi.org/10.1016/j.diabres.2011.10.029
Fu, Z.; Gilbert, E. R.; Liu, D. Curr. Diabetes Rev. 2013, 9, 25-53. DOI: https://doi.org/10.2174/157339913804143225
Brannmark, C.; Nyman, E.; Fagerholm, S.; Bergenholm, L.; Ekstrand, E.; Cedersund, G.; Stralfors, P. J. Biol. Chem. 2013, 288, 9867-9880. DOI: http://dx.doi.org/10.1074/jbc.M112.432062 DOI: https://doi.org/10.1074/jbc.M112.432062
Tangvarasittichai, S. World J. Diabetes. 2015, 6, 456-80. DOI: http://dx.doi.org/10.4239/wjd.v6.i3.456 DOI: https://doi.org/10.4239/wjd.v6.i3.456
Farrugia, G.; Balzan, R. Fron. Oncol. 2012, 2, 64-64. DOI: http://dx.doi.org/10.3389/fonc.2012.00064 DOI: https://doi.org/10.3389/fonc.2012.00064
Cossarizza, A.; Ferraresi, R.; Troiano, L.; Roat, E.; Gibellini, L.; Bertoncelli, L.; Nasi, M.; Pinti, M. Nat. Protoc. 2009, 4, 1790-1797. DOI: http://dx.doi.org/10.1038/nprot.2009.189 DOI: https://doi.org/10.1038/nprot.2009.189
Siddique, Y. H.; Ara, G.; Afzal, M. Dose-Response. 2012, 10, 1-10. DOI: http://dx.doi.org/10.2203/dose-response.10-002.Siddique DOI: https://doi.org/10.2203/dose-response.10-002.Siddique
Fernandes, A. C. F.; Melo, J. B.; Genova, V. M.; Santana, A. L.; Macedo, G. Recent Pat. Food, Nutr. Agric. 2022, 13, 3-16. DOI: http://dx.doi.org/10.2174/2212798412666210528130001 DOI: https://doi.org/10.2174/2212798412666210528130001
Imam, M. U.; Musa, S. N. A.; Azmi, N. H.; Ismail, M. Int. J. Mol. Sci. 2012, 13, 12952-12969. DOI: http://dx.doi.org/10.3390/ijms131012952 DOI: https://doi.org/10.3390/ijms131012952
Sung, M.; Park, S. S.; Kim, S.; Han, C.; Hur, J. Food Sci. Biotechnol. 2014, 23, 1615-1621. DOI: http://dx.doi.org/10.1007/s10068-014-0220-3 DOI: https://doi.org/10.1007/s10068-014-0220-3
Balkan, B. M.; Kismali, G.; Alpay, M.; Sayiner, S.; Turan, D.; Balkan, A. B.; Salmanoglu, B.; Karagul, H.; Sel, T. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2016, 22, 865-869. DOI: http://dx.doi.org/10.9775/kvfd.2016.15499 DOI: https://doi.org/10.9775/kvfd.2016.15499
Chen, W.; Shaw, L.; Chang, P.; Tung, S.; Chang, T.; Shen, C.; Hsieh, Y.; Wei, K. Experimental and Therapeutic Medicines. 2016, 11, 1231-1238. DOI: http://dx.doi.org/10.3892/etm.2016.3077 DOI: https://doi.org/10.3892/etm.2016.3077
Sriset, Y.; Chatuphonprasert, W.; Jarukamjorn, K. Tropical J. Pharm. Res. 2019, 18, 1001-1007. DOI: http://dx.doi.org/10.4314/tjpr.v18i5.13 DOI: https://doi.org/10.4314/tjpr.v18i5.13
Lee, H.; Lim, Y. J. Nutr. Biochem. 2018, 57, 77-85. DOI: http://dx.doi.org/10.1016/j.jnutbio.2018.03.016 DOI: https://doi.org/10.1016/j.jnutbio.2018.03.016
Polce, S. A.; Burke, C.; Franca, L. M.; Kramer, B.; de Andrade Paes, A. M.; Carrillo-Sepulveda, M. A. Nutrients. 2018, 10. DOI: http://dx.doi.org/10.3390/nu10050531 DOI: https://doi.org/10.3390/nu10050531
Balabolkin, M. L.; Klebanova, E. M. Terapevticheskii. 2003, 75, 72-77.
Taylor, R. Journal of the Royal College of Physicians of Edinburgh. 2017, 47, 168-171. DOI: http://dx.doi.org/10.4997/JRCPE.2017.216 DOI: https://doi.org/10.4997/JRCPE.2017.216
Li, C.; He, J.; Zhou, X.; Xu, X. China Journal of Chinese Materia Medica. 2017, 42, 2254-2260. DOI: http://dx.doi.org/10.19540/j.cnki.cjcmm.20170307.014
Schmidt, M. I.; Bracco, P. A.; Duncan, B. B. Lancet Diabetes Endocrinol. 2019, 7, 424. DOI: http://dx.doi.org/10.1016/S2213-8587(19)30148-2 DOI: https://doi.org/10.1016/S2213-8587(19)30148-2
Jamal, P.; Barkat, A. A.; Amid, A. Afr. J. Biotechnol. 2011, 10, 18788-18794. DOI: http://dx.doi.org/10.5897/AJB11.2754 DOI: https://doi.org/10.5897/AJB11.2754
Han, H.; Li, Z.; Gao, Z.; Yin, X.; Dong, P.; Yang, B.; Kuang, H. Nat. Prod. Res. 2019, 33, 2845-2850. DOI: http://dx.doi.org/10.1080/14786419.2018.1508143 DOI: https://doi.org/10.1080/14786419.2018.1508143
Chen, W.; Shen, Y.; Su, H.; Zheng, X. Chem.-Biol. Interact. 2014, 219, 83-89. DOI: http://dx.doi.org/10.1016/j.cbi.2014.05.010 DOI: https://doi.org/10.1016/j.cbi.2014.05.010
Floegel, A.; Kim, D.; Chung, S.; Koo, S. I.; Chun, O. K. J. Food Compos. Anal. 2011, 24, 1043-1048. DOI: http://dx.doi.org/10.1016/j.jfca.2011.01.008 DOI: https://doi.org/10.1016/j.jfca.2011.01.008
Chi, C.; Hu, F.; Wang, B.; Li, T.; Ding, G. J. Funct. Foods. 2015, 15, 301-313 DOI: http://dx.doi.org/10.1016/j.jff.2015.03.045 DOI: https://doi.org/10.1016/j.jff.2015.03.045
Vijayalakshmi, M.; Ruckmani, K. Bangladesh Journal of Pharmacology. 2016, 11, 570-572. DOI: http://dx.doi.org/10.3329/bjp.v11i3.27663 DOI: https://doi.org/10.3329/bjp.v11i3.27663
Yilmazer-Musa, M.; Griffith, A. M.; Michels, A. J.; Schneider, E.; Frei, B. J. Agric. Food Chem. 2012, 60, 8924-8929. DOI: http://dx.doi.org/10.1021/jf301147n DOI: https://doi.org/10.1021/jf301147n
Kazeem, M. I.; Adamson, J. O.; Ogunwande, I. A. BioMed Research International. 2013, 2013. DOI: http://dx.doi.org/10.1155/2013/527570 DOI: https://doi.org/10.1155/2013/527570

Downloads
Published
Issue
Section
License
Copyright (c) 2023 Jingya Liu, Yinqiu Zheng, Shuang Dai, Li Li , Wei Wu, Rong Gou, Deyuan Wang, Shiyu Long, Meihua Huang, Zhihong Xu

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









