Chemical Constituents from Artemisia annua and Vitex agnus-castus as New Aromatase Inhibitors: In-vitro and In-silico Studies
DOI:
https://doi.org/10.29356/jmcs.v64i4.1236Keywords:
Aromatase inhibitors, Artemisia annua L. and Vitex agnus-castus L., β-sitosterol, myricetin-3,7,4'-trimethyl ether, domesticosideAbstract
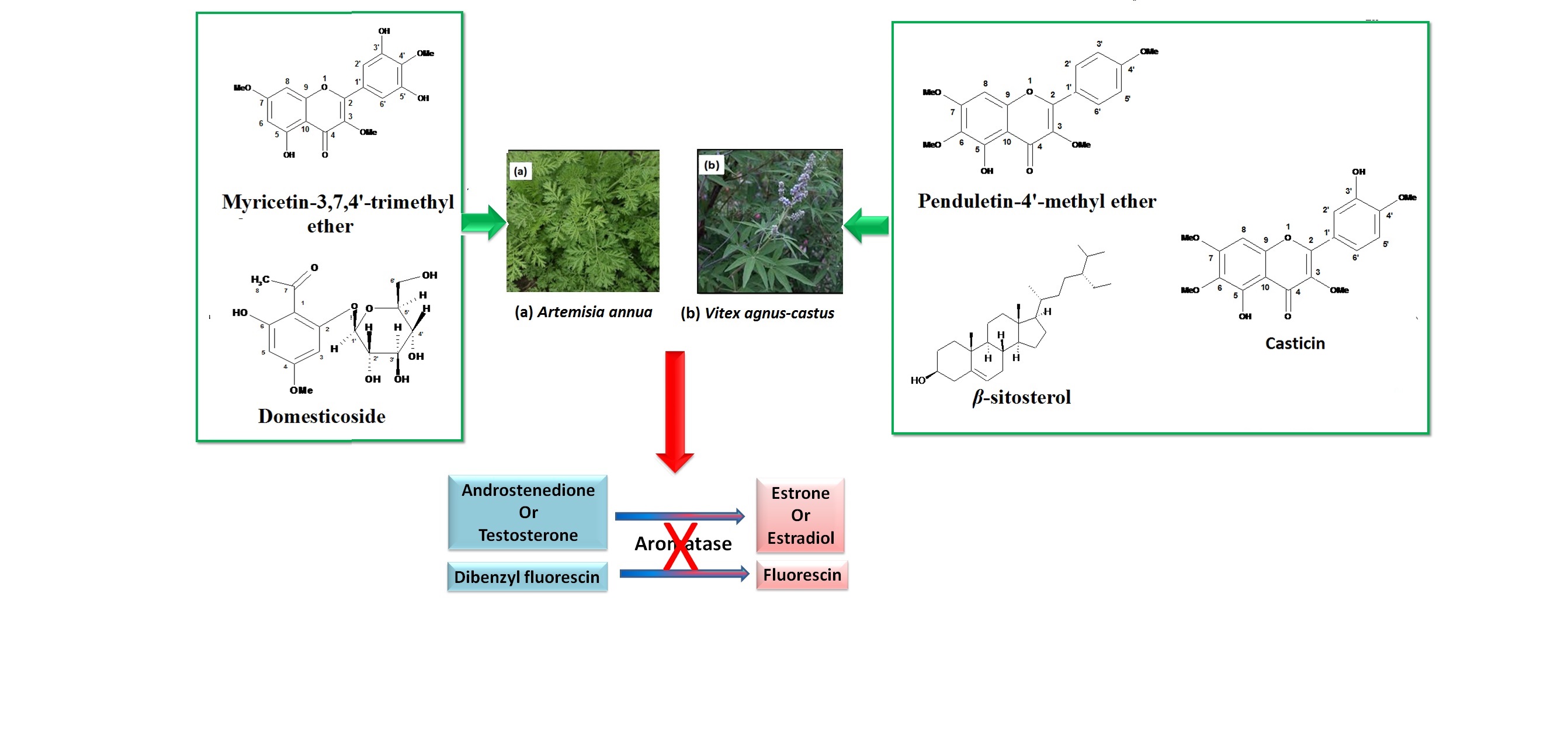
Abstract. Aromatase inhibitors are important in certain cancers such as breast cancer in postmenopausal women. In this study, eight constituents from Artemisia annua L. and Vitex agnus-castus L. were isolated and evaluated for their aromatase inhibitory activity using in-vitro fluorimetric assay. All tested compounds possessed moderate to strong inhibitory activity with β-sitosterol and myricetin-3,7,4'-trimethyl ether being the most active with IC50 values of 0.13 and 0.25 μM, respectively. Compounds were subjected to induced fit docking (IFD) where β-sitosterol, possessed comparable interaction patterns to the natural co-crystallized ligand androstenedione. Furthermore, Absorption, Distribution, Metabolism, Excretion and Toxicity (ADME&T) properties of the compounds were evaluated revealing that all compounds' properties - except some of β- sitosterol related to solubility - lied within the acceptable range for human use, thereby considered as competent drug-like molecules. These findings could qualify β- sitosterol, myricetin-3,7,4'-trimethyl ether and domesticoside as lead compounds for the development of new aromatase inhibitors.
Downloads
References
Jiang, H.; Shi, J.; Li, Y. Molecules 2011, 16, 3146-3151. DOI: https://doi.org/10.3390/molecules16043146
Balunas, M. J.; Su, B.; Brueggemeier, R. W.; Kinghorn, A. D. Anti-cancer Agents Med. Chem. 2008, 8, 646-682. DOI: https://doi.org/10.2174/1871520610808060646
Balunas, M. J.; Kinghorn, A. D. Planta Med. 2010, 76, 1087. DOI: https://doi.org/10.1055/s-0030-1250169
Ryu, J.-H.; Lee, S.-J.; Kim, M.-J.; Shin, J.-H.; Kang, S.-K.; Cho, K.-M.; Sung, N.-J. J. Korean Soc. Food Sci. Nutr. 2011, 40, 509-516. DOI: https://doi.org/10.3746/jkfn.2011.40.4.509
Al-Zubaidy, A. A. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 303-306.
Kaushik, A. C.; Kumar, S.; Wei, D. Q.; Sahi, S. Front. Chem. 2018, 6. DOI: https://doi.org/10.3389/fchem.2018.00023
Kumavath, R.; Azad, M.; Devarapalli, P.; Tiwari, S.; Kar, S.; Barh, D.; Azevedo, V.; Kumar, A. P. Bioinformation. 2016, 12, 324-331. DOI: https://doi.org/10.6026/97320630012324
Tripathi, S. K.; Selvaraj, C.; Singh, S. K.; Reddy, K. K. Med. Chem. Res. 2012, 21, 4239-4251. DOI: https://doi.org/10.1007/s00044-011-9940-6
Hend M. Dawood, R. S. I., Eman Shawky a, Hala M. Hammoda, Aly M. Metwally, Development of a validated HPTLC-bioautographic method for evaluation of aromatase inhibitory activity of plant extracts and their constituents: comparative quantitation using peak area and reciprocal iso-inhibition volume methods.
(a) Semenya, S.; Maroyi, A.; Potgieter, M.; Erasmus, L. Afr. J. Tradit., Complementary Altern. Med. 2013, 10, 331-339; (b) Raja, R. R. Afr. J. Plant Sci. 2015, 9, 320-326.
Stresser, D. M., High-Throughput Screening of Human Cytochrome P450 Inhibitors Using Fluorometric Substrates: Methodology for 25 Enzyme/Substrate Pairs, Humana Press?, 2004, 215-230. DOI: https://doi.org/10.1385/1-59259-800-5:215
Bisswanger, H. Perspect. Sci. 2014, 1, 41-55. DOI: https://doi.org/10.1016/j.pisc.2014.02.005
Schrödinger, L., QikProp 3.0 User Manual. 2007.
Blaskó, G.; Cordell, G. A.; Lankin, D. C. Journal Nat. Prod. 1988, 51, 1273-1276. DOI: https://doi.org/10.1021/np50060a040
Yu, S.; Fang, N.; Mabry, T. J. Phytochemistry. 1987, 26, 2131-2133. DOI: https://doi.org/10.1016/S0031-9422(00)81782-3
Brown, G. D.Molecules. 2010, 15, 7603-98. DOI: https://doi.org/10.3390/molecules15117603
Delnavazi, M.-R.; Hadjiakhoondi, A.; Delazar, A.; Ajani, Y.; Yassa, N., Azerosides A and B: Two new phloroacetophenone glycosides from the roots of Dorema glabrum Fisch. & C.A. Mey, Vol. 24, 2014. DOI: https://doi.org/10.1007/s00044-014-1138-2
Wollenweber, E.; Schober, I.; Dennis, C. W.; George, Y. Phytochemistry. 1985, 24, 2129-2131. DOI: https://doi.org/10.1016/S0031-9422(00)83141-6
Shaikh, A.; Makhmoor, T.; Choudhary, M., Radical scavenging potential of compounds isolated from Vitex agnus-castus, Vol. 34, 2010, 119.
Sarojini, K.; Krishnan, H. Rom. J. Biophy. 2014, 24.
Halgren, T. A.; Murphy, R. B.; Friesner, R. A.; Beard, H. S.; Frye, L. L.; Pollard, W. T.; Banks, J. L. J. Med. Chem. 2004, 47, 1750-1759. DOI: https://doi.org/10.1021/jm030644s
Pirhadi, S.; Ghasemi, J. B. Mol. Inf. 2012, 31, 856-866. DOI: https://doi.org/10.1002/minf.201200018
Kalani, K.; Yadav, D. K.; Khan, F.; Srivastava, S. K.; Suri, N. J. Mol. Model. 2012, 18, 3389-3413. DOI: https://doi.org/10.1007/s00894-011-1327-6
Hosen, S. Z.; Dash, R.; Khatun, M.; Akter, R.; Bhuiyan, M. H. R.; Rezaul, M.; Karim, N. J. M.; Ahamed, F.; Islam, K. S.; Afrin, S. J. Appl. Pharm. Sci. 2017, 7, 120-128. DOI: https://doi.org/10.7324/JAPS.2017.70116
Ntie-Kang, F. SpringerPlus. 2013, 2, 353. DOI: https://doi.org/10.1186/2193-1801-2-353
Khan, M. F.; Verma, G.; Akhtar, W. Arabian J. Chem. 2016.

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.