The Theoretical Study on the Mechanism of [3+2] Cycloaddition Reactions between α,β-unsaturated Selenoaldehyde with Nitrone and with Nitrile Oxide
Mechanism of 1,3-dipolar cycloaddition reactions
DOI:
https://doi.org/10.29356/jmcs.v64i2.1111Keywords:
Selenoaldehyde, nitrone, nitrile oxide, MP2, ELF, cycloaddition, MEDTAbstract
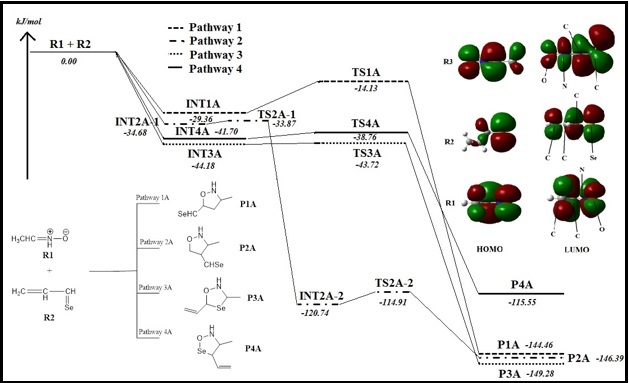
Abstract. The reaction mechanisms of [3+2] cycloaddition (32CA) between the α,β-unsaturated selenoaldehyde with nitrone and nitrile oxide were investigated theoretically using the molecular electron density theory (MEDT). Selenoaldehyde has two unsaturations which allow for the cycloaddition occurring. It was expected to undergo four regioisomeric reaction paths in two separate reactions with nitrone and nitrile oxide. The study was conducted using ab initio approach at MP2/6-31G(d) level of theory. Potential energy surfaces were generated from the energies of the stationary points involved in the mechanisms and the dominant reaction pathways were identified. It was found that Pathway 3 and 4 are the two competing reaction channels, where the cycloaddition reaction occurs at the selenium-analogue carbonyl group of selenoaldehyde. The reactivity indices were analysed at the ground state of the reactants to predict the reactivity of studied organic molecules in 32CA reactions. Analysis of the electronic structure of nitrone and nitrile oxide, the three-atom-components (TACs), and their participation in 32CA reactions towards selenoaldehyde allows establishing a useful classification of 32CA reactions into zwitterionin-type (zw-type) reactions involving TACs with a high zwitterionic character.
Resumen. Se estudia teóricamente, utilizando la teoría de la densidad electrónica molecular (MEDT), el mecanismo de reacción de la cicloadición [3+2] (32CA) entre selenoaldehídos α, β insaturados con nitrona y óxido de nitrilo. El selenoaldehído tiene dos insaturaciones que permiten la cicloadición. Se esperaba que la reacción se llevara a cabo a lo largo de cuatro caminos regioisoméricos en dos reacciones separadas con la nitrona y el óxido de nitrilo. Se realizó un estudio ab initio con el nivel de teoría MP2/6-31G(d). Se generaron superficies de energía potencial a partir de las energías de los puntos estacionarios involucrados en el mecanismo y se identificaron los caminos de reacción dominantes. Se encontró que dos rutas, la 3 y la 4, son los canales de reacción que compiten para que ocurra la cicloadición en el grupo carbonilo análogo al selenio del selenoaldheído. Se analizaron los índices de reactividad de los estados basales de los reactivos para predecir la reactividad de las moléculas orgánicas estudiadas en las reacciones 32CA. El análisis de la estructura electrónica de la nitrona y el óxido de nitrilo, de las componentes triatómicas (TACs) y de su participación en las reacciones 32CA hacia el selenoaldheído permite clasificar a las reacciones 32CA en tipo zwitteriónico (zw) que involucran a los TACs con un elevado carácter zwitteriónico.
Downloads
References
Liu, K.C.; Howe, R.K. J. Org. Chem. 1983, 48, 4590-4592. DOI: https://doi.org/10.1021/jo00172a030
Lacy, C.; Scheuer, P. J. J. Nat. Prod. 2000, 63, 119-12. DOI: https://doi.org/10.1021/np9902643
Liu, S.; Fu, X.; Scheuer, F. J.; Kelly-Borges, P. J. J. Nat. Prod. 1997, 60, 614-615. DOI: https://doi.org/10.1021/np970070s
Ichiba, T.; Scheuer, P. J. J. Org. Chem. 1993, 58, 4149-4150. DOI: https://doi.org/10.1021/jo00067a062
Brandi, A.; Cicchi, S.; Cordero, F. M.; Goti, A. Chem. Rev. 2003, 103, 1213-1270. DOI: https://doi.org/10.1021/cr010005u
Kanemasa, S.; Tsuge, O. Heterocycles 1990, 30, 719-736. DOI: https://doi.org/10.3987/REV-89-SR3
King, S. W.; Riordan, J. M.; Holt, E. M.; Stammer, C. H. J. Org. Chem. 1982, 47, 3270-3273. DOI: https://doi.org/10.1021/jo00138a014
Padwa, A. 1,3-Dipolar Cycloaddition Chemistry; Wiley: New York, 1984.
Gothelf, K. V.; Jørgensen, K. A. Chem. Rev. 1998, 98, 863-910. DOI: https://doi.org/10.1021/cr970324e
Karlsson, S.; Högberg, H. E. Org. Prep. Proced. Int. 2001, 33, 103-172. DOI: https://doi.org/10.1080/00304940109356583
Pellissier, H. Tetrahedron 2007, 63, 3235-3285. DOI: https://doi.org/10.1016/j.tet.2007.01.009
Confalone, P. N.; Huie, E. M. Org. React. 1988, 36, 1-173. DOI: https://doi.org/10.1007/BF02071159
Black, D.; Crozier, R. F.; Davies, V. C. Synthesis 1975, 205-221. DOI: https://doi.org/10.1055/s-1975-23713
Ding, P.; Miller, M.; Chen, Y.; Helquist, P.; Oliver, A. J.; Wiest, O. Org. Lett. 2004, 6, 1805-1808. DOI: https://doi.org/10.1021/ol049473r
Wess, G.; Kramer, W.; Schubert, G.; Enhsen, A.; Baringhaus, K. H.; Globmik, H.; Müller, S.; Bock, K.; Klein, H.; John, M.; Neckermann, G.; Hoffmann, A. Tetrahedron Lett. 1993, 34, 819-822. DOI: https://doi.org/10.1016/0040-4039(93)89021-H
Padwa, A.; Pearson, W. H. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Wiley & Sons: New York, 2002. DOI: https://doi.org/10.1002/0471221902
Zhao, H.; Li, X.; Ran, X.; Zhang, W. J. Mol. Struct. (Theochem) 2004, 683, 207-213. DOI: https://doi.org/10.1016/j.theochem.2004.06.019
Nacereddine, A. K.; Yahia, W.; Bouacha, S.; Djerourou, A. Tetrahedron Lett. 2010, 51, 2617-2621. DOI: https://doi.org/10.1016/j.tetlet.2010.03.025
Dorostkar-Ahmadi, N.; Bakavoli, M.; Moeinpour, F.; Davoodnia, A. Spectrochim. Acta A 2011, 79, 1375-1380. DOI: https://doi.org/10.1016/j.saa.2011.04.071
Magnuson, E. C.; Pranata, J. J. Comput. Chem. 1998, 19, 1795-1804. DOI: https://doi.org/10.1002/(SICI)1096-987X(199812)19:16<1795::AID-JCC1>3.0.CO;2-G
Cossío, F. P.; Morao, I.; Jiao, H.; Schleyer, P. J. Am. Chem. Soc. 1999, 121, 6737-6746. DOI: https://doi.org/10.1021/ja9831397
Rastelli, A.; Gandolfi, R.; Sarzi-Amande, M.; Carboni, B. J. Org. Chem. 2001, 66, 2449-2458. DOI: https://doi.org/10.1021/jo001801n
Di Valentin, C.; Freccero, M.; Gandolfi, R.; Rastelli, A. J. Org .Chem. 2000, 65, 6112-6120. DOI: https://doi.org/10.1021/jo000569i
Domingo, L. R. Eur. J. Org. Chem. 2000, 2000, 2265-2272. DOI: https://doi.org/10.1002/1099-0690(200006)2000:12<2265::AID-EJOC2265>3.0.CO;2-C
Carda, M.; Portolés, R.; Murga, J.; Uriel, S.; Marco, J. A.; Domingo, L. R.; Zaragozá, R. J.; Röper, H. Eur. J. Org. Chem. 2000, 65, 7000-7009. DOI: https://doi.org/10.1021/jo0009651
Merino, P.; Revuelta, J.; Tejero, T.; Chiacchio, U.; Rescifina, A.; Romeo, G. Tetrahedron 2003, 59, 3581-5392. DOI: https://doi.org/10.1016/S0040-4020(03)00547-7
Fleming, I. Frontiers Orbitals and Organic Chemical Reactions, Wiley, London, 1976.
Houk, K. N. Acc. Chem. Res. 1975, 8, 361-369. DOI: https://doi.org/10.1021/ar50095a001
Kang, K. H.; Pae, A. N.; Choi, K. I.; Cho, Y. S.; Chung, B. Y.; Lee, J. E.; Jung, S. H.; Koh, H. Y.; Lee, H. Y. Tetrahedron Lett. 2001, 42, 1057-1060. DOI: https://doi.org/10.1016/S0040-4039(00)02180-8
Shang, Y. J.; Wang, Y. G. Synthesis 2002, 2002, 1663-1668. DOI: https://doi.org/10.1055/s-2002-33655
Shankar, B. B.; Yang, D. Y.; Girton, S.; Ganguly, A. K. Tetrahedron Lett. 1998, 39, 2447-2448. DOI: https://doi.org/10.1016/S0040-4039(98)00237-8
Kumar, R. S.; Rajesh, S. M.; Perumal, S.; Yogeeswari, P.; Sriram, D. Tetrahedron Asymm. 2010, 21, 1315-1327. DOI: https://doi.org/10.1016/j.tetasy.2010.03.054
Chen, S.; Ren, J.; Wang, Z. Tetrahedron 2009, 65, 9146-9151. DOI: https://doi.org/10.1016/j.tet.2009.09.034
Okazaki, R. Yuki Gosei Kagaku Kyokaishi 1988, 46, 1149-1163. DOI: https://doi.org/10.5059/yukigoseikyokaishi.46.1149
Tokitoh, N.; Okazaki, R. Pol. J. Chem. 1998, 72, 971-1000.
Rohr, U.; Schatz, J.; Sauer, J. Eur. J. Org. Chem. 1998, 2875-2883. DOI: https://doi.org/10.1002/(SICI)1099-0690(199812)1998:12<2875::AID-EJOC2875>3.3.CO;2-E
Bachrach, S. M.; Jiang, S. J. Org. Chem. 1999, 64, 8248-8255. DOI: https://doi.org/10.1021/jo991000o
Liao, H. Y.; Su, M. D.; Chu, S. Y. Chem. Phys. 2000, 261, 275-287. DOI: https://doi.org/10.1016/S0301-0104(00)00286-X
Orlova, G.; Goddard, J. D. J. Org. Chem. 2001, 66, 4026-4035. DOI: https://doi.org/10.1021/jo010280g
Litvinov, V. P.; Dyachenko, V. D. Russ. Chem. Rev. 1997, 66, 923-951. DOI: https://doi.org/10.1070/RC1997v066n11ABEH000323
Segi, M.; Nakajima, T.; Suga, S.; Murai, S.; Ryu, I.; Ogawa, A.; Sonoda, N. J. Am. Chem. Soc. 1988, 110, 1976-1978. DOI: https://doi.org/10.1021/ja00214a058
Segi, M.; Takahashi, M.; Nakajima, T.; Suga, S.; Murai, S.; Sonoda, N. Tetrahedron Lett. 1988, 29, 6965-6968. DOI: https://doi.org/10.1016/S0040-4039(00)88488-9
Segi, M.; Koyama, T.; Nakajima, T.; Suga, S.; Murai, S.; Sonoda, N. Tetrahedron Lett. 1989, 30, 2095-2098. DOI: https://doi.org/10.1016/S0040-4039(01)93721-9
Segi, M.; Kato, M.; Nakajima, T. Tetrahedron Lett. 1991, 32, 7427-7430. DOI: https://doi.org/10.1016/0040-4039(91)80125-P
Li, G. M.; Segi, M.; Nakajima, T. Tetrahedron Lett. 1992, 33, 3515–3518. DOI: https://doi.org/10.1016/S0040-4039(00)92677-7
Segi, M.; Takahashi, T.; Ichinose, H.; Li, G. M.; Nakajima, T. Tetrahedron Lett. 1992, 33, 7865-7868. DOI: https://doi.org/10.1016/S0040-4039(00)74764-2
Li, G. M.; Niu, S.; Segi, M.; Zingaro, R. A,; Yamamoto, H.; Watanabe, K.; Nakajima, T.; Hall, M. B. J. Org. Chem. 1999, 64, 1565-1575. DOI: https://doi.org/10.1021/jo982028n
Segi, M. Yuki Gosei Kagaku Kyokaishi 2003, 61, 661-669. DOI: https://doi.org/10.5059/yukigoseikyokaishi.61.661
Segi, M.; Tanno, K.; Kojima, M.; Honda, M.; Nakajima, T. Tetrahedron Lett. 2007, 48, 2303-2306. DOI: https://doi.org/10.1016/j.tetlet.2007.01.153
Petersson, G. A.; Bennett, A.; Tensfeldt, T. G.; Al-Laham, M. A.; Shirley, W. A.; Mantzaris, J. J. Chem. Phys. 1988, 89, 2193-2218. DOI: https://doi.org/10.1063/1.455064
Petersson, G. A.; Al-Laham, M. A. J. Chem. Phys. 1991, 94, 6081-6090. DOI: https://doi.org/10.1063/1.460447
(a) Frisch, M. J.; Head-Gordon, M.; Pople, J. A. Chem. Phys. Lett. 1990, 166, 275-280.; (b); Chem. Phys. Lett. 1990, 166, 281-289.; (c) Head-Gordon, M.; Pople, J. A.; Frisch, M. J. Chem. Phys. Lett. 1988, 153, 503-506.; (d) Head-Gordon M. T. Chem. Phys. Lett. 1994, 220, 122-128.; (e) Saebø, S.; Almlöf, J. Chem. Phys. Lett. 1989, 154, 83-89.
Frisch, J. et al., Gaussian, Inc., Wallingford CT, 2016. Gaussian 09, Revision.
Parr, R. G.; Szentpály, L. v.; Liu, S., J. Am. Chem. Soc. 1999, 121, 1922-1924. DOI: https://doi.org/10.1021/ja983494x
Parr, R. G.; Pearson, R. G., J. Am. Chem. Soc. 1983, 105, 7512-7516. DOI: https://doi.org/10.1021/ja00364a005
Parr, R. G.; Weitao, Y., Oxford University Press: 1994.
Kohn, W.; Sham, L. J., Phys. Rev. 1965, 140, A1133-A1138. DOI: https://doi.org/10.1103/PhysRev.140.A1133
Domingo, L. R.; Chamorro, E.; Pérez, P., ?J. Org. Chem. 2008, 73, 4615-4624. DOI: https://doi.org/10.1021/jo800572a
Domingo, L. R.; Pérez, P.; Sáez, J. A., RSC Adv. 2013, 3, 1486-1494. DOI: https://doi.org/10.1039/C2RA22886F
Domingo, L. R.; Aurell, M. J.; Pérez, P.; Contreras, R., Tetrahedron. 2002, 58, 4417-4423. DOI: https://doi.org/10.1016/S0040-4020(02)00410-6
Jaramillo, P.; Domingo, L. R.; Chamorro, E.; Pérez, P., J. Mol. Struct.: THEOCHEM 2008, 865, 68-72. DOI: https://doi.org/10.1016/j.theochem.2008.06.022
Domingo, L., Molecules. 2016, 21, 1319. DOI: https://doi.org/10.3390/molecules21101319
Domingo, L. R.; Sáez, J. A., J. Org. Chem. 2010, 76, 373-379. DOI: https://doi.org/10.1021/jo101367v
Domingo, R. L.; Ríos-Gutiérrez, M.; Acharjee, N., Molecules. 2019, 24. DOI: https://doi.org/10.3390/molecules24050832

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








