The effect of tamoxifen on the electrochemical behavior of Bi+3/Bi on the glassy carbon electrode and its determination via differential pulse anodic stripping voltammetry
DOI:
https://doi.org/10.29356/jmcs.v63i1.689Keywords:
bismuth film electrode, tamoxifen, glassy carbon electrodes, differential pulse anodic stripping voltammetry, serum and pharmaceutical samplesAbstract
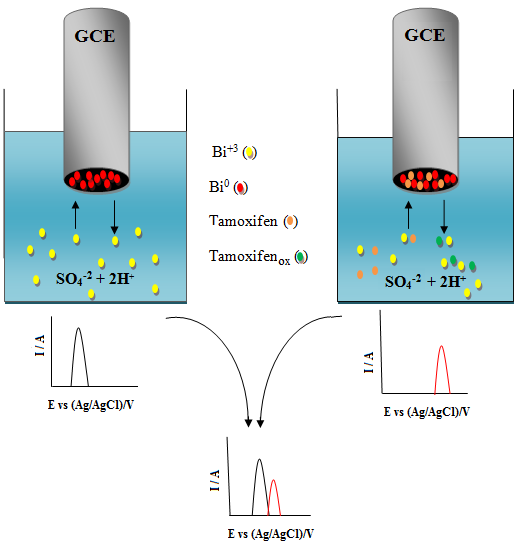
The electrochemical behavior of Bi+3 ions on the surface of a glassy carbon electrode, in acidic media and in the presence of tamoxifen, was investigated. Cyclic voltammetry, chronoamperometry, differential pulse voltammetry, electrochemical impedance spectroscopy, and scanning electron microscopy with energy-dispersive X-ray spectroscopy were used to find the probable mechanism contributing to the reduction of the peak height of bismuth oxidation with an increase in the concentration of tamoxifen. The obtained results show a slight interaction between the bismuth species and tamoxifen which co-deposit on the surface of glassy carbon electrode. Therefore, the reduction in the peak height of bismuth oxidation as a function of tamoxifen concentration was used to develop a new differential pulse anodic striping voltammetry method for determination of trace amount of tamoxifen. The effects of experimental parameters on the in situ DPASV of Bi+3 ions in the presence of tamoxifen shown the optimal conditions as: 2 mol L-1 H2SO4 (1% v v-1 MeOH), a deposition potential of -0.5 V, a deposition time of 60 s, and a glassy carbon electrode rotation rate of 300 rpm. The calibration curve was plotted in the range of 0.5 to 6 µg mL-1 and the limits of detection and quantitation were calculated to be 3.1 × 10-5 µg mL-1 and 1.0 × 10-4 µg mL-1, respectively. The mean, RSD, and relative bias for 0.5 µg mL-1 (n=5) were found to be 0.49 µg mL-1, 0.3%, and 2%, respectively. Finally, the proposed method was successfully used for the determination of tamoxifen in serum and pharmaceutical samples.
Downloads
References
Naseri, N. G.; Baldock, S. J; Economou, A.; Goddard, N. J.; Fielden, P. R. Anal Bioanal Chem. 2008, 391, 1283-1292. DOI: https://doi.org/10.1007/s00216-008-1948-5
Lu, D.; Wang, J.; Ninivin, C. L.; Mabic, S.; Dimitrakopoulos, T. J Electroanal Chem. 2011, 651, 46-49. DOI: https://doi.org/10.1016/j.jelechem.2010.11.002
Figueiredo-Filho, L. C. S. d.; Santos, V. B. D.; Janegitz, B. C.; Guerreiro, T. B.; Fatibello-Filho. O.; Faria, R. C.; Marcolino-Junior, L. H. Electroanalysis. 2010, 22, 1260-1266. DOI: https://doi.org/10.1002/elan.200900553
Wang, J.; Lu, J. Electrochem Commun. 2000, 2, 390-393. DOI: https://doi.org/10.1016/S1388-2481(00)00045-X
Svancara, I.; Fairouz, M.; Ismail, K.; Metelka, R.; Vytras, K. Sci Pap Univ Pardubice Ser A. 2003, 9, 31-48.
Wang, J.; Lu, J. M.; Ho?evar, S. B.; Farias, P. A. M.; Ogorevc. B. Anal Chem. 2000, 72, 3218-3222. DOI: https://doi.org/10.1021/ac000108x
Guzsvány, V.; Kádár, M.; Papp, Z.; Bjelica. L.; Gaál. F.; Tóth, K. Electroanalysis. 2008, 20, 291-300. DOI: https://doi.org/10.1002/elan.200704057
Campestrini, I.; Braga, O. C. d.; Vieira, I. C.; Spinelli, A. Electrochim Acta. 2010. 55. 4970-4975. DOI: https://doi.org/10.1016/j.electacta.2010.03.105
Luo, L.; Wang, X.; Ding, Y.; Li, Q.; Jia, J.; Deng, D. Anal Methods. 2010, 2, 1095-1100. DOI: https://doi.org/10.1039/c0ay00150c
Ho?evar, S. B.; Ogorevc, B.; Wang. J.; Pihlar. B. Electroanalysis. 2002, 14, 1707-1712. DOI: https://doi.org/10.1002/elan.200290014
Pauliukaite, R.; Metelka, R.; Švancara, I.; Królika, A.; Bobrowski, A.; Vyt?as, K.; Norkus, E.; Kalcher, K. Anal Bioanal Chem. 2002, 374, 1155-1158. DOI: https://doi.org/10.1007/s00216-002-1569-3
Baldo, M. A.; Daniele, S.; Bragato, C. J Phys IV France. 2003, 107, 103-106. DOI: https://doi.org/10.1051/jp4:20030254
Tesarova, E.; Heras, A.; Colina, A.; Ruiz, V.; Švancara, I.; Vyt?as, K.; Lopez-Palacios, J. Anal Chim Acta. 2008, 608, 140-146. DOI: https://doi.org/10.1016/j.aca.2007.12.023
Ho?evar, S. B.; Švancara, I., Vyt?as, K.; Ogorevc, B. Electrochim Acta. 2005, 51, 706-710. DOI: https://doi.org/10.1016/j.electacta.2005.05.023
Falahieh, Z. D.; Jalali, M.; Alimoradi, M. Turk. J Chem. 2017, 41, 826-844. DOI: https://doi.org/10.3906/kim-1612-25
Sastry, C. S. P.; Rao, J. S. V. M. L.; Rao, K. R. Talanta. 1995, 42, 1479-1485. DOI: https://doi.org/10.1016/0039-9140(95)01598-6
Sane, R. T.; Desai, S. V.; Sonawne, K. K.; Nayak, V. G. J Chromatogr A. 1985, 331, 432-436. DOI: https://doi.org/10.1016/0021-9673(85)80052-2
Heath, D. D.; Flatt, S. W.; Wu, A. H. B.; Pruitt, M. A.; Rock, C. L. Br J Biomed Sci. 2014, 71, 33–39. DOI: https://doi.org/10.1080/09674845.2014.11669960
Thang, L. Y.; See, H. H.; Quirino, J. P. Electrophoresis. 2016, 37, 1166-1169. DOI: https://doi.org/10.1002/elps.201600010
Fijalek, Z.; Chodkowski, J.; Warowna, M. J Electroanal Chem. 1987, 226, 129-136. DOI: https://doi.org/10.1016/0022-0728(87)80038-4
Radhapyari, K.; Kotoky, P.; Khan, R. Mater Sci Eng C. 2013, 33, 583–587. DOI: https://doi.org/10.1016/j.msec.2012.09.021
Jalali, M.; Aliakbar, A. Anal Methods. 2013, 5, 6352-6359. DOI: https://doi.org/10.1039/c3ay41264d
Flores, J. R.; Nevado, J. J. B.; Salcedo, A. M. C.; Díaz, M. P. C. Anal Chim Acta. 2004, 512, 287-295. DOI: https://doi.org/10.1016/j.aca.2004.02.048
El-Leithy, E. S.; Abdel-Rashid, R. S. Asian J Pharm Sci. 2016, 11, 318-325. DOI: https://doi.org/10.1016/j.ajps.2016.02.005
Peris-Vicente, J.; Carda-Broch, S.; Esteve-Romero, J. Anal Sci. 2014, 30, 925-930. DOI: https://doi.org/10.2116/analsci.30.925
Santana, D. P. d.; Brage, R. M. C.; Strattmman, R.; Albuquerque, M. M.; Bedor, D. C. G.; Leal, L. B.; Silva, J. A. d. Quím Nova. 2008, 31, 47-52. DOI: https://doi.org/10.1590/S0100-40422008000100010
Kanberoglu, G. S.; Coldur, F.; ?ubuk, O.; Topcu, C.; Caglar, B. Sens Lett. 2016, 14, 59-64. DOI: https://doi.org/10.1166/sl.2016.3568
Wang, J.; Cai, X.; Fernandes, J. R.; Ozsoz, M.; Grant, D. H. Talanta. 1997, 45, 273-278. DOI: https://doi.org/10.1016/S0039-9140(97)00129-X
Jain, R.; Vikas, Radhapyari, K. Drug Test Analysis. 2011, 3, 743-747. DOI: https://doi.org/10.1002/dta.240

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









