Methylene: The Linker of Two Aromatic Iminium Salts
DOI:
https://doi.org/10.29356/jmcs.v63i3.525Keywords:
Aromatic iminium salts, Pi-conjugated triazene, X-ray structure, iodomethane, methylene chloride.Abstract
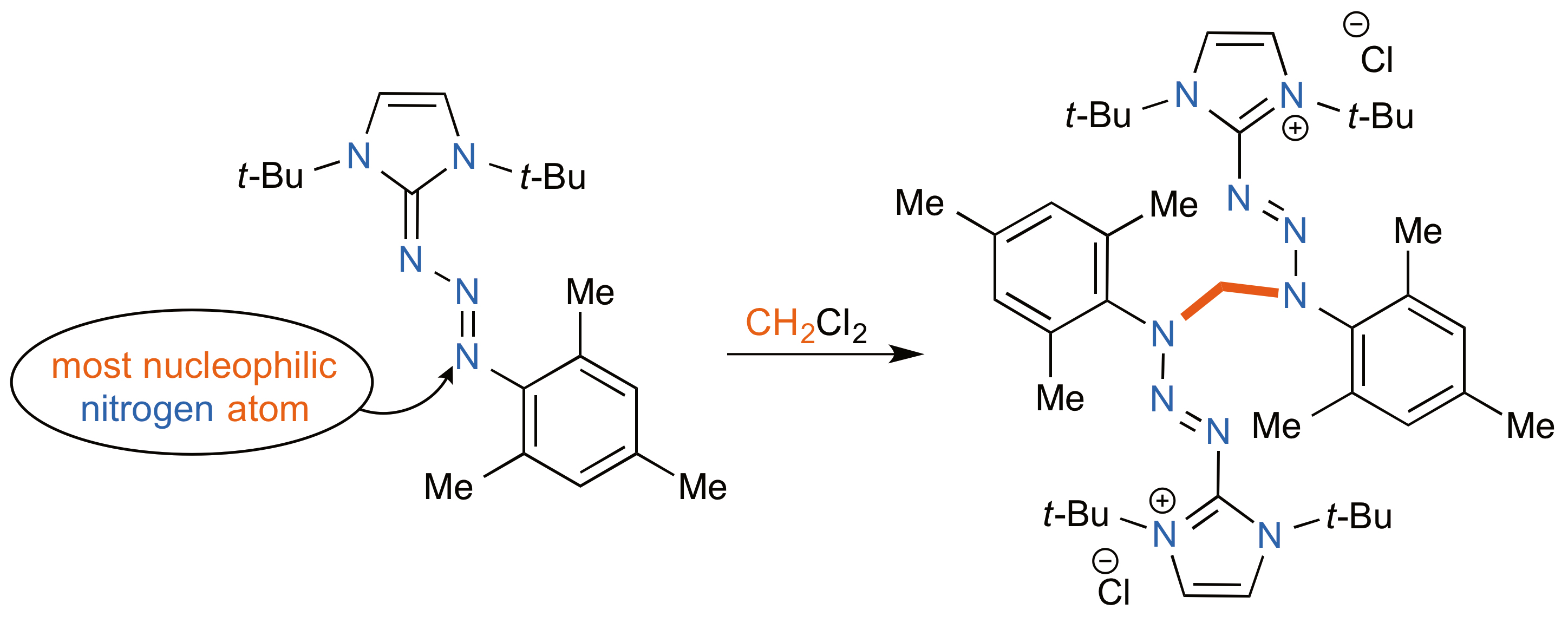
A straightforward synthesis of aromatic iminium salts has been developed by coupling 2-Azido-1,3,5-trimethyl benzene with 1,3-ditert-butylimidazolium tetrafluoroborate in basic conditions, followed by treatment with dichloromethane or iodomethane. Herein, we report the synthetic procedure and full characterization data, including X-ray structure analysis, of the expected bis(triazenyl)methane adduct 5. Moreover, we have discovered what constitutes a double carbon-chlorine bond activation.
Downloads
References
Howell, J. M.; Feng, K.; Clark, J. R.; Trzepkowski, L. J.; White, M. C. J. Am. Chem. Soc. 2015, 137, 14590-14593. DOI: https://doi.org/10.1021/jacs.5b10299
Andrez, J.-C. Beilstein J. Org. Chem. 2009, 5, 33. DOI: https://doi.org/10.3762/bjoc.5.33
Baumann, M.; Baxendale, I. R.; Ley, S. V.; Nikbin, N. Beilstein J. Org. Chem. 2011, 7, 442-495. DOI: https://doi.org/10.3762/bjoc.7.57
Assary, R. S.; Brushett, F. R.; Curtiss, L. A. RSC Adv. 2014, 4, 57442-57451. DOI: https://doi.org/10.1039/C4RA08563A
Chiba, S. Synlett 2012, 2012, 21-44. DOI: https://doi.org/10.1055/s-0031-1290108
Patil, S.; White, K.; Bugarin, A. Tetrahedron Lett. 2014, 55, 4826–4829. DOI: https://doi.org/10.1016/j.tetlet.2014.07.022
Patil, S.; Bugarin, A. Acta Crystallogr., Sect. E: Struct. Rep. Online 2014, 70, 224–227 DOI: https://doi.org/10.1107/S1600536814020698
Patil, S.; Bugarin, A. Eur. J. Org. Chem. 2016, 2016, 860?870. DOI: https://doi.org/10.1002/ejoc.201501218
Pogoreltsev, A.; Tulchinsky, Y.; Fridman, N.; Gandelman, M. J. Am. Chem. Soc. 2017, 139, 4062-4067. DOI: https://doi.org/10.1021/jacs.6b12360
Back, J.; Park, J.; Kim, Y.; Kang, H.; Kim, Y.; Park, M. J.; Kim, K.; Lee, E. J. Am. Chem. Soc. 2017, 139, 15300-15303. DOI: https://doi.org/10.1021/jacs.7b08753
Barragan, E.; Bugarin, A. J. Org. Chem. 2017, 82, 1499–1506. DOI: https://doi.org/10.1021/acs.joc.6b02705
Barragan, E.; Noonikara-Poyil, A.; Yang, C-H.; Wang, H.; Bugarin, A. Org. Chem. Front. 2019, 6, 152–161. DOI: https://doi.org/10.1039/C8QO00938D
Zincsuk, J.; Carnavale, G. A. Arkivoc 2016, 4, 352–362. DOI: https://doi.org/10.3998/ark.5550190.p009.567
Pattacini, R.; Jie, S.; Braunstein, P. Chem. Commun. 2009, 890-892. DOI: https://doi.org/10.1039/b817728g
Simmons, H. E.; Smith, R. D. J. Am. Chem. Soc. 1958, 80, 5323-5324. DOI: https://doi.org/10.1021/ja01552a080
Rossberg, M. , Lendle, W. , Pfleiderer, G. , Tögel, A. , Dreher, E. , Langer, E. , Rassaerts, H. , Kleinschmidt, P. , Strack, H. , Cook, R. , Beck, U. , Lipper, K. , Torkelson, T. R., Löser, E. , Beutel, K. K. and Mann, T. (2006). Chlorinated Hydrocarbons. In Ullmann's Encyclopedia of Industrial Chemistry, (Ed.). DOI: https://doi.org/10.1002/14356007.a06_233.pub2
Archer, R. H.; Zones, S. I.; Davis, M. E. Microporous Mesoporous Mater. 2010, 130, 255-265. DOI: https://doi.org/10.1016/j.micromeso.2009.11.018
Lu, Y.; Wang, L.; Wang, X.; Xi, T.; Liao, J.; Wang, Z.; Jiang, F. Eur. J. Med. Chem., 2017, 135, 125-141. DOI: https://doi.org/10.1016/j.ejmech.2017.04.040
For additional crystallography data of compound 5 in CIF and other formats, see the CCDC 1832942.

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.