First Report on Cultivated Salvia hispanica in an Arid Climate: UPLC-MS/MS Analysis, Antioxidant, and Enzymatic Activities
DOI:
https://doi.org/10.29356/jmcs.v70i1.2485Keywords:
Salvia hispanica, polyphenols, UPLC-MS/MS, antioxidant activity, α-amylase inhibitionAbstract
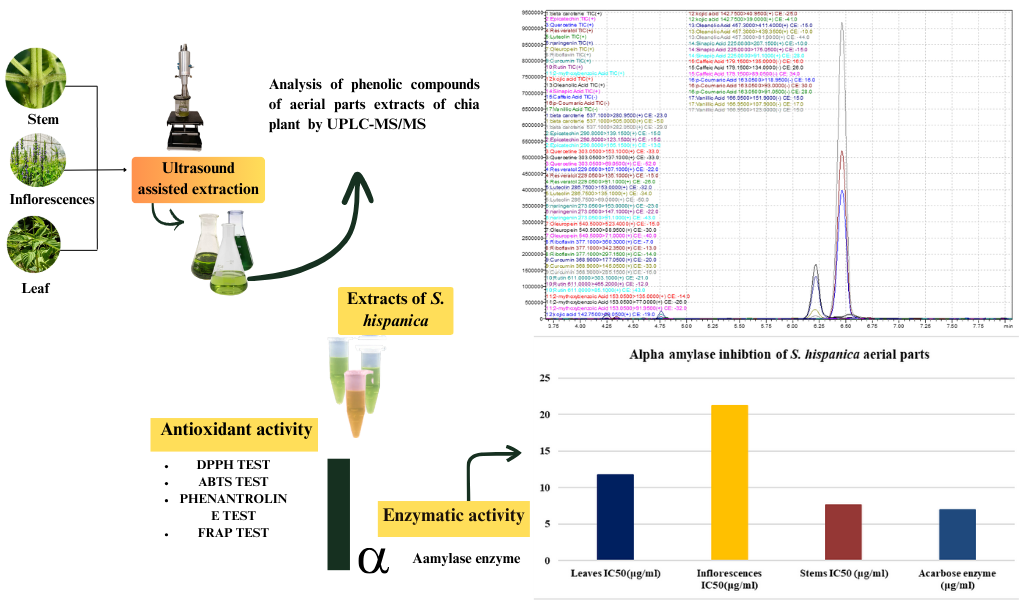
Abstract. This study is the first to identify polyphenols and flavonoids in ethanolic extracts of aerial parts of Salvia hispanica cultivated under arid conditions using UPLC–MS analysis. Antioxidant activity was evaluated through DPPH, ABTS, PHE, and FRAP tests, while enzymatic activity was assessed via α-amylase inhibition. The polyphenol and flavonoid contents were highest in the inflorescences, followed by the leaves and stems. The major compounds identified included oleanolic acid, riboflavin, rutin, naringenin, curcumin, caffeic acid, resveratrol, quercetin, epicatechin, and luteolin. The chelating activity of leaf, inflorescences, and stem extracts ranged from 11.97 to 145.86 μg mL-1, while α-amylase inhibitory activity ranged from 7.66 to 21.25 μg mL-1. These findings highlight the pharmacological potential of S. hispanica, particularly its antioxidant and antidiabetic properties. This research enhances scientific understanding of Chia’s aerial parts, supporting the development of plant-based supplements and medicinal applications for health and wellness.
Resumen. Este estudio es el primero en identificar polifenoles y flavonoides en extractos etanólicos de las partes aéreas de Salvia hispanica cultivadas en condiciones áridas mediante análisis UPLC-MS. La actividad antioxidante se evaluó mediante pruebas DPPH, ABTS, PHE y FRAP, mientras que la actividad enzimática se evaluó mediante inhibición de la α-amilasa. Los contenidos de polifenoles y flavonoides fueron más altos en las inflorescencias, seguidos de las hojas y los tallos. Los principales compuestos identificados incluyeron ácido oleanólico, riboflavina, rutina, naringenina, curcumina, ácido cafeico, resveratrol, quercetina, epicatequina y luteolina. La actividad quelante de los extractos de hojas, inflorescencias y tallos varió de 11.97 a 145.86 μg mL-1, mientras que la actividad inhibidora de α-amilasa varió de 7.66 a 21.25 μg mL-1. Estos hallazgos resaltan el potencial farmacológico de S. hispanica, en particular sus propiedades antioxidantes y antidiabéticas. Esta investigación profundiza la comprensión científica de las partes aéreas de la chía, impulsando el desarrollo de suplementos vegetales y aplicaciones medicinales para la salud y el bienestar.
Downloads
References
1. Henry, H. S.; Mittleman, M.; Mc Crohan, P. R., in: Introducción de la chía y lagoma de tragacanto en los Estados Unidos. En: Avances en Cosechas Nuevas. Prensa de la Madera. Portland, Ohio, O. J. Janick y J. E. Simon: 1990, 252–256.
2. Ayerza, R.; Coates, W. Ann. Nutr. Metab. 2007, 51, 27–34. DOI: https://doi.org/10.1159/000100818
3. Baginsky, C.; Arenas, J.; Escobar, H.; Garrido, M.; Valero, N.; Tello, D.; Pizarro, L.; Valenzuela, A.; Morales, L.; Silva, H. Chil. J. Agric. Res. 2016, 76, 255–264. DOI: https://doi.org/10.4067/S0718-58392016000300001
4. Orona-Tamayo, D.; Valverde, M. E.; Paredes-López, O., in: Sustainable Protein Sources; Elsevier: 2017, 265–281. DOI: https://doi.org/10.1016/B978-0-12-802778-3.00017-2
5. Basuny, A. M.; Arafat, S. M.; Hikal, D. M. Food Nutr. Sci. 2021, 12, 479–493. DOI: https://doi.org/10.4236/fns.2021.126037
6. Silva, L. D. A.; Verneque, B. J. F.; Mota, A. P. L.; Duarte, C. K. Food Funct. 2021, 12, 8835–8849. DOI: https://doi.org/10.1039/d1fo01287h
7. Khalid, W.; Arshad, M. S.; Aziz, A.; Rahim, M. A.; Qaisrani, T. B.; Afzal, F.; Ali, A.; Ranjha, M. M. A. N.; Khalid, M. Z.; Anjum, F. M. Food Sci. Nutr. 2023, 11, 3–16. DOI: https://doi.org/10.1002/fsn3.3035
8. Agarwal, A.; Rizwana; Tripathi, A. D.; Kumar, T.; Sharma, K. P.; Patel, S. K. S. Antioxidants. 2023, 12, 1413. DOI: https://doi.org/10.3390/antiox12071413
9. Borneo, R.; Aguirre, A.; León, A. E. J. Am. Diet. Assoc. 2010, 110, 946–949. DOI: https://doi.org/10.1016/j.jada.2010.03.011
10. Das, A. Adv. Biotechnol. Microbiol. 2017, 5, 555661.
11. Ixtaina, V. Y.; Martínez, M. L.; Spotorno, V.; Mateo, C. M.; Maestri, D. M.; Diehl, B. W. K.; Nolasco, S. M.; Tomás, M. C. J. Food Compos. Anal. 2011, 24, 166–174. DOI: https://doi.org/10.1016/j.jfca.2010.08.006
12. Muñoz, L. A.; Cobos, A.; Diaz, O.; Aguilera, J. M. Food Rev. Int. 2013, 29, 394–408. DOI: https://doi.org/10.1080/87559129.2013.818014
13. Toscano, L. T.; da Silva, C. S. O.; Toscano, L. T.; de Almeida, A. E. M.; da Cruz Santos, A.; Silva, A. S. Plant Foods Hum. Nutr. 2014, 69, 392–398. DOI: https://doi.org/10.1007/s11130-014-0452-7
14. Scapin, G.; Schmidt, M. M.; Prestes, R. C.; Rosa. Int. Food Res. J. 2016, 23, 2341–2346.
15. Kechebar, A.; Karoune, S.; Falleh, H.; Belhamra, M.; Rahmoune, C.; Ksouri, R. Courr. Savoir. 2017, 23, 29–38.
16. Vuksan, V.; Jenkins, A. L.; Brissette, C.; Choleva, L.; Jovanovski, E.; Gibbs, A. L.; Bazinet, R. P.; Au-Yeung, F.; Zurbau, A.; Ho, H. V. T.; Duvnjak, L.; Sievenpiper, J. L.; Josse, R. G.; Hanna, A. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146. DOI: https://doi.org/10.1016/j.numecd.2016.11.124
17. Mihafu, F. D.; Kiage, B. N.; Kimang’a, A. N.; Okoth, J. K. Int. J. Biol. Chem. Sci. 2020, 14, 1752–1762. DOI: https://doi.org/10.4314/ijbcs.v14i5.20
18. Tamargo, A.; Martin, D.; Navarro del Hierro, J.; Moreno-Arribas, M. V.; Muñoz, L. A. Food Res. Int. 2020, 137, 109364. DOI: https://doi.org/10.1016/j.foodres.2020.109364
19. Felemban, L. F.; Attar, A. M. A.; Zeid, I. M. A. J. Pharm. Res. Int. 2021, 41, 15–26. https://doi.org/10.9734/jpri/2020/v32i4131040
20. Rabail, R.; Khan, M. R.; Mehwish, H. M.; Rajoka, M. S. R.; Lorenzo, J. M.; Kieliszek, M.; Khalid, A. R.; Shabbir, M. A.; Aadil, R. M. Front. Biosci. 2021, 26, 643–654. DOI: https://doi.org/10.3390/molecules27185907
21. Rahmoune, I.; Karoune, S.; Azzam, C.; Saad, S.; Foughalia, A.; Sarri, M.; Chebrouk, F.; Abidat, H.; Kechebarre, M. S. A. Agr. Acad. J. 2024, 7, 19–33. DOI: https://doi.org/10.32406/v7n5/2024/19-33/agrariacad
22. Elshafie, H. S.; Aliberti, L.; Amato, M.; De Feo, V.; Camele, I. Eur. Food Res. Technol. 2018, 244, 1675–1682. DOI: https://doi.org/10.1007/s00217-018-3080-x
23. Dziadek, K.; Kopeć, A.; Dziadek, M.; Sadowska, U.; Cholewa-Kowalska, K. Molecules. 2022, 27, 1569. DOI: https://doi.org/10.3390/molecules27051569
24. Abdel Ghani AE, Al-Saleem MSM, Abdel-Mageed WM, AbouZeid EM, Mahmoud MY, Abdallah RH. Mdpi. 2023, 12,1062. DOI: https://doi.org/10.3390/plants12051062
25. Motyka, S.; Kusznierewicz, B.; Ekiert, H.; Korona-Głowniak, I.; Szopa, A. Molecules 2023, 28, 2728. DOI: https://doi.org/10.3390/molecules28062728
26. Maturana, G.; Segovia, J.; Olea-Azar, C.; Uribe-Oporto, E.; Espinosa, A.; Zúñiga-López, M. C. Antioxidants. 2023, 12, 1108. DOI: https://doi.org/10.3390/antiox12051108
27. Huang, M.; Xu, H.; Zhou, Q.; Xiao, J.; Su, Y.; Wang, M. Crit. Rev. Food Sci. Nutr. 2024, 15, 1–23. DOI: https://doi.org/10.1080/10408398.2024.2337220
28. Herman, S.; Marco, G.; Cecilia, B.; Alfonso, V.; Luis, M.; Cristián, V.; Sebastián, P.; Sebastián, A. Agric. Water Manag. 2016, 173, 67–75. DOI: https://doi.org/10.1016/j.agwat.2016.04.028
29. Müller, L.; Gnoyke, S.; Popken, A. M.; Böhm, V. LWT. 2010, 43, 992–999. DOI: https://doi.org/10.1016/j.lwt.2010.02.004
30. Topçu, G.; Ay, M.; Bilici, A.; Sarikürkcü, C.; Öztürk, M.; Ulubelen, A. Food Chem. 2007, 103, 816–822. DOI: https://doi.org/10.1016/j.foodchem.2006.09.028
31. Bouchoukh, I.; Hazmoune, T.; Boudelaa, M.; Bensouici, C.; Zellagui, A. Curr. Issues Pharm. Med. Sci. 2019, 32, 160–167.
32. Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Free Radic. Biol. Med. 1999, 26, 1231–1237. DOI: https://doi.org/10.1016/S0891-5849(98)00315-3
33. Oyaizu, M. Jpn. J. Nutr. 1986, 44, 307–315.
34. Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Talanta. 2008, 76, 899–905. DOI: https://doi.org/10.1016/j.talanta.2008.04.055
35. Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. Ind. Crops Prod. 2014, 53, 244–251. DOI: https://doi.org/10.1016/j.indcrop.2013.12.043
36. Aminu, A.; Idris, H.; Muhammad, A.; Aliyu, B.; Namadina, M.; Abdulkadir, A.; Adetutu, E.; Zango, U.; Abdulkadir, S.; Kutama, R.; et al. Biol. Environ. Sci. J. Trop. 2023, 20, 158–176. DOI: https://doi.org/10.4314/bestj.v20i3.16
37. Hanganu, D.; Olah, N. K.; Pop, C. E.; Vlase, L.; Oniga, I.; Ciocarlan, N.; Matei, A.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Benedec, D. Farmacia. 2019, 67, 801–805. DOI: https://doi.org/10.31925/farmacia.2019.5.8
38. Svydenko, L. Agrobiodivers. Improv. Nutr. Health Life Qual. 2022, 6, 139–148. DOI: https://doi.org/10.15414/ainhlq.2022.0015
39. Nasr, A.; Yosuf, I.; Turki, Z.; Abozeid, A. BMC Plant Biol. 2023, 23. DOI: https://doi.org/10.1186/s12870-023-04341-5
40. Mahdjoub, M. M.; Benzitoune, N.; Maiz, Y.; Aouadi, N. E. H.; Bouhenna, M. M.; Kadri, N. J. Res. Pharm. 2023, 27, 1076–1085. DOI: https://doi.org/10.29228/jrp.400
41. Amato, M.; Caruso, M. C.; Guzzo, F.; Galgano, F.; Commisso, M.; Bochicchio, R.; Labella, R.; Favati, F. Eur. Food Res. Technol. 2015, 241, 615–625. DOI: https://doi.org/10.1007/s00217-015-2488-9
42. Ilioara, O.; Laurian, V.; Daniela, H.; Anca, T.; Daniela, B. Hop Med. Plants. 2018, 26, 77–84. DOI: https://doi.org/10.15835/hpm.v26i1-2.13235
43. Luca, S. V.; Skalicka-Woźniak, K.; Mihai, C. T.; Gradinaru, A. C.; Mandici, A.; Ciocarlan, N.; Miron, A.; Aprotosoaie, A. C. Antioxidants. 2023, 12, 1514. DOI: https://doi.org/10.3390/antiox12081514
44. Anah, U.; Offer, S.; Aniekan, S.; Nkechi, J.; Romanus, A.; Olorunfemi, A. Niger. J. Pharm. Appl. Sci. Res. 2024, 13, 65–75. DOI: https://doi.org/10.60787/nijophasr-v13-i1-535
45. Olfat, N.; Ashoori, M.; Saedisomeolia, A. Br. J. Nutr. 2022, 128, 1887–1895. DOI: https://doi.org/10.1017/S0007114521005031
46. Horii, S.; Hiroshi, F.; Takao, M.; Yukihiko, K.; Naoki, A.; Katsuhiko, M. J. Med. Chem. 1986, 29, 1038–1046. DOI: https://doi.org/10.1021/jm00156a023
47. Mutlu, M.; Bingol, Z.; Ozden, E. M.; Köksal, E.; Erturk, A.; Goren, A. C.; Alwasel, S.; Gulcin, İ. Electron J. Biotechnol. 2025, 74, 41–53. DOI: https://doi.org/10.1016/j.ejbt.2024.12.002
48. Banu, S. A.; John, S.; Jane Monica, S. Res. J. Pharm. Technol. 2021, 12, 6289–6294. DOI: https://doi.org/10.52711/0974-360X.2021.01088
49. Javid, H.; Moein, S.; Moein, M. Clin. Phytosci. 2022, 8, 7. DOI: https://doi.org/10.1186/s40816-022-00339-y
50. Mamache, W.; Amira, S.; Ben Souici, C.; Laouer, H.; Benchikh, F. J. Food Biochem. 2020, 44, e13472. DOI: https://doi.org/10.1111/jfbc.13472
51. Papoutsis, K.; Zhang, J.; Bowyer, M. C.; Brunton, N.; Gibney, E. R.; Lyng, J. Food Chem. 2021, 338, 128119. DOI: https://doi.org/10.1016/j.foodchem.2020.128119
52. Visvanathan, R.; Le, D. T.; Dhital, S.; Rali, T.; Davis, R. A.; Williamson, G. J. Med. Chem. 2024, 67, 18753–18763. DOI: https://doi.org/10.1021/acs.jmedchem.4c01042
53. Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki, D. S.; Rahbar, S. Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani, J. N.; Raeesi, S. Phytother. Res. 2021, 35, 1719–1738. DOI: https://doi.org/10.1002/ptr.6904
54. Saeed, R.; Ahmed, D. Microchem. J. 2024, 9, 1-7. DOI: https://doi.org/10.1016/j.microc.2024.110622.
55. Meigui, H.; Qiao, X.; Yonghong, L.; Mehraj, A.; Jiajia, T.; Qiuhong, L.; Chen, T. Food Biosci. 2024, 61, 104951. DOI: https://doi.org/10.1016/j.fbio.2024.104951
56. Asmaey, M.; Elsoghiar, A.; Shaaban, M. Chem. Afr. 2024, 7, 5123–5148. DOI: https://doi.org/10.1007/s42250-024-01110-1

Downloads
Published
Issue
Section
License
Copyright (c) 2025 Ibtissem Rahmoune, Samira Karoune, Clara Azzam, Somia Saad, Noui Hendel, Hadji Toka, Farid Chebrouk, Thamere Cheriet, Mohamed SeifAllah Kechebar, Ouahiba Mizab

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








