A Practical and Instructive Approach to Purify Acetonitrile for a Wide Electrochemical Window
DOI:
https://doi.org/10.29356/jmcs.v67i4.2013Keywords:
Acetonitrile purification, electrolyte purification, electrochemical window, nonaqueous electrolytes, CH3CNAbstract
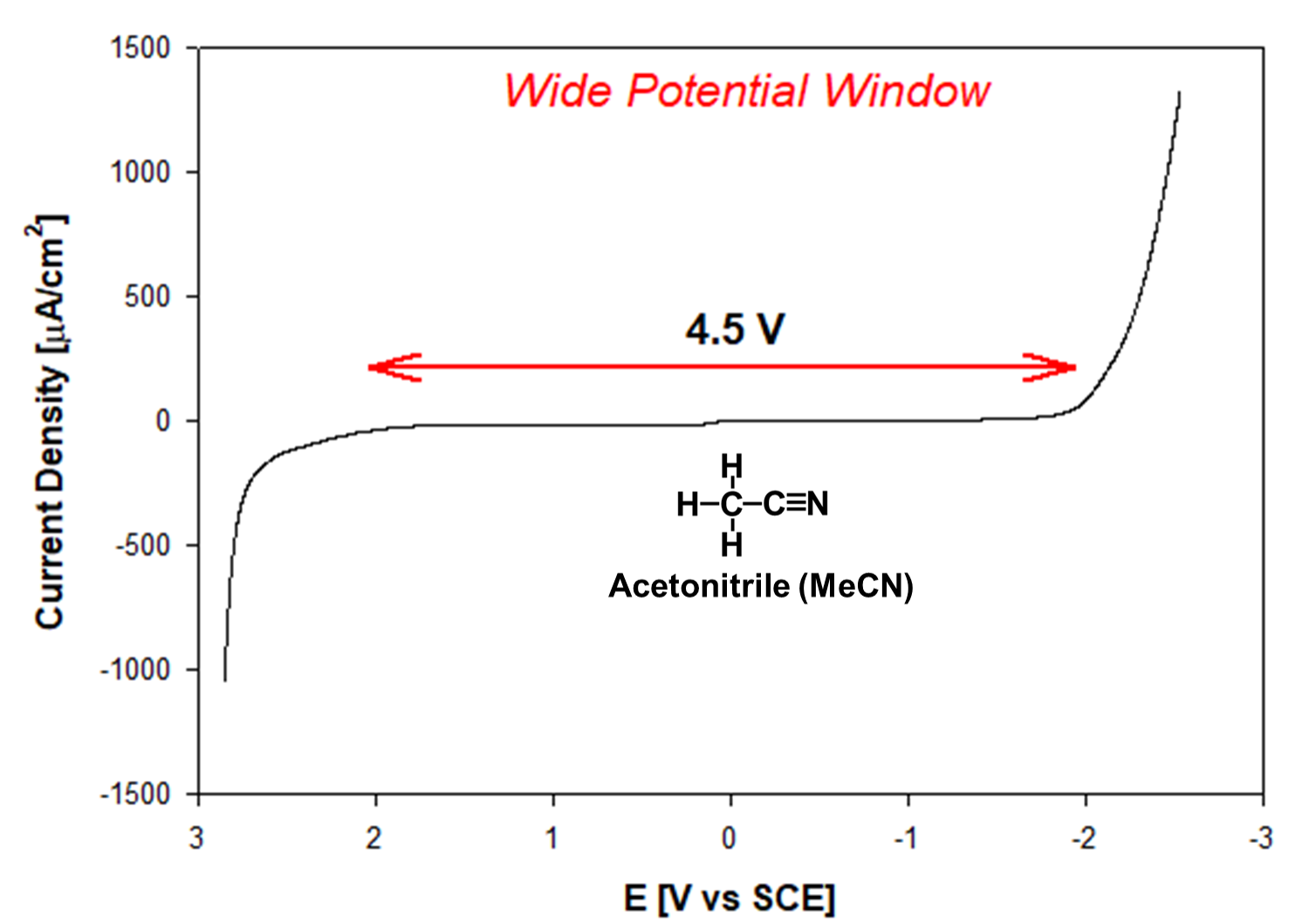
Abstract. Because of its large electrochemical window, acetonitrile (MeCN) is one of the most widely used solvents in electrochemistry. It is a suitable solvent for nonaqueous electrolytes that allows studies of cathodic and anodic processes, but electrolyte purification remains challenging. As received, the high-performance liquid chromatography (HPLC) grade is unsuitable for most electroanalytical applications. We present an approach to optimize the purification of HPLC-grade acetonitrile to yield a tetrabutylammonium perchlorate (TBAP)/MeCN electrolyte for experiments in nonaqueous media. We used cyclic voltammetry (CV) to show the background due to impurities and to guide the experimental design to a background current acceptable for CVs of a 1 mM typical concentration of a redox-active molecule. We use 3A molecular sieves, followed by distillation over CaH2 with a final treatment with Al2O3. The optimized procedure yields CH3CN with small background currents, increasing the signal-to-noise ratio and minimizing chemical complications over a wide potential window. Our approach includes discriminating between impurities in the solvent and electrolyte salts; for TBAP, we recrystallize from ethyl acetate and 95 % ethanol. The process and theoretical guidelines apply to other nonaqueous electrolytes dealing with electroactive impurities, including organic molecules, oxygen, and water.
Resumen. El acetonitrilo (MeCN) es uno de los disolventes más utilizados en electroquímica por su amplia ventana electroquímica. Es un disolvente adecuado para electrolitos no acuosos que permite estudiar procesos electroquímicos catódicos y anódicos. Sin embargo, para llevar a cabo estos estudios, es importante considerar la purificación del electrolito que sigue siendo un reto. Tal como se recibe, el acetonitrilo para cromatografía de alto rendimiento (HPLC, por las siglas en inglés) es inadecuado para la mayoría de las aplicaciones electroanalíticas. En este trabajo presentamos un método para optimizar la purificación del acetonitrilo grado HPLC y obtener un electrolito de perclorato de tetrabutilamonio, (TBAP)/MeCN. Utilizamos voltametría cíclica para determinar la corriente de fondo y diseñar una corriente residual aceptable para una concentración típica de 1 mM para una molécula con actividad electroquímica. Utilizamos tamices moleculares 3Å, seguidos de destilación sobre CaH2 y finalizamos con un tratamiento en Al2O3. El procedimiento optimizado produce CH3CN con pequeñas corrientes de fondo, aumentando la relación señal-ruido y minimizando las complicaciones químicas en una amplia ventana de potencial. Nuestro enfoque incluye la discriminación entre las impurezas del disolvente y en las sales del electrolito. Recristalizamos el TBAP con una mezcla de acetato de etilo y etanol al 95 %. El proceso y las directrices teóricas se pueden aplicar a otros electrolitos no acuosos con impurezas electroactivas, como moléculas orgánicas, oxígeno y agua.
Downloads
References
Mann, C. K., in: Electroanal. Chem, Bard, A. J., Ed. Vol 3, Marcel Dekker, New York 1969, 57-134
Fry, A. J., in: Laboratory techniques in Electroanalytical Chemistry, Kissinger, P. T.; Heineman, W. R., Eds.; Marcel Dekker: New York, 2007, 469-485. DOI: https://doi.org/10.1201/9781315274263.
Creager, S., in: Handbook of Electrochemistry, Zoski, C. G., Ed., Elsevier, Amsterdam, 2007, 57- 72. DOI: https://doi.org/10.1016/B978-0-444-51958-0.X5000-9. DOI: https://doi.org/10.1016/B978-044451958-0.50004-5
Cao, W.; Zhang, X.; Bard, A. J. J. Electroanal. Chem. 2004, 566, 409-413 DOI: https://doi.org/10.1016/j.jelechem.2003.11.054.
Yang, W.; Kazemi, R. R.; Karunathilake, N.; Catalano, V. J.; Alpuche-Aviles, M. A.; Chalifoux, W. Org. Chem. Front. 2018, 5, 2288-2295. DOI: 10.1039/C8QO00389K. DOI: https://doi.org/10.1039/C8QO00389K
Bard, A. J.; Faulkner, L. R.; White, H. S., in: Electrochemical Methods: Fundamentals and Applications, 3rd ed.; John Wiley & Sons: New York, 2022, 1044.
Coetzee, J. F.; Cunningham, G. P.; McGuire, D. K.; Padmanabhan, G. R. Anal. Chem. 1962, 34, 1139-1143. DOI: 10.1021/ac60189a034. DOI: https://doi.org/10.1021/ac60189a034
Lund H., in: Organic Electrochemistry. Lund, H.; Hammerich, O., Eds.; Marcel Dekker, Inc., New York, 2001, 223-292. DOI: https://doi.org/10.1201/9781420029659.
Perrin, D. D.; Armarego, W. L. F.; Perrin, D. R., in: Purification of Laboratory Chemicals, 2nd ed.; Pergamon Press: Oxford, 1980, 79-81.
Williams, D. B. G.; Lawton, M. J. Org. Chem. 2010, 75, 8351-8354. DOI: 10.1021/jo101589h. DOI: https://doi.org/10.1021/jo101589h
Burfield, D. R.; Lee, K.-H.; Smithers, R. H. J. Org. Chem. 1977, 42, 3060-3065. DOI: 10.1021/jo00438a024. DOI: https://doi.org/10.1021/jo00438a024
Pangborn, A. B.; Giardello, M. A.; Grubbs, R. H.; Rosen, R. K.; Timmers, F. J. Organometallics. 1996, 15, 1518-1520. DOI: 10.1021/om9503712. DOI: https://doi.org/10.1021/om9503712
Alaimo, P. J.; Peters, D. W.; Arnold, J.; Bergman, R. G. J. Chem. Educ. 2001, 78, 64. DOI: 10.1021/ed078p64. DOI: https://doi.org/10.1021/ed078p64
Rashid, M. U.; Tahir, Z.; Kim, S.; Jang, J. I.; Kim, Y. S. ACS Omega. 2021, 6, 31366-31374. DOI: 10.1021/acsomega.1c05348. DOI: https://doi.org/10.1021/acsomega.1c05348
Miller, T. A.; Prater, B.; Lee, J. K.; Adams, R. N. J. Am. Chem. Soc. 1965, 87, 121-122. DOI: 10.1021/ja01079a023. DOI: https://doi.org/10.1021/ja01079a023
Gangadharan, A.; Mamidi, S.; Sharma, C. S.; Rao, T. N. Mater. Today Commun. 2020, 23, 100926. DOI: https://doi.org/10.1016/j.mtcomm.2020.100926.
Bharti, V. K.; Gangadharan, A.; Kumar, S. K.; Pathak, A. D.; Sharma, C. S. Mater. Adv. 2021, 2, 3031-3041. DOI: 10.1039/D1MA00115A. DOI: https://doi.org/10.1039/D1MA00115A
Baek, H.-M.; Kim, D.-Y.; Lee, W.-J.; Kang, J. RSC Advances. 2020, 10, 36478-36484. DOI: 10.1039/D0RA07195A. DOI: https://doi.org/10.1039/D0RA07195A
Kakunuri, M.; Sharma, C. S. Electrochim. Acta. 2015, 180, 353-359. DOI: https://doi.org/10.1016/j.electacta.2015.08.124.
Saini, D.; Gunture; Kaushik, J.; Aggarwal, R.; Tripathi, K. M.; Sonkar, S. K. ACS Appl. Nano Mater.2021, 4, 12825-12844. DOI: 10.1021/acsanm.1c02840. DOI: https://doi.org/10.1021/acsanm.1c02840
Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. Sustainable Energy Fuels. 2020, 4, 5387-5416. DOI: 10.1039/D0SE00175A. DOI: https://doi.org/10.1039/D0SE00175A

Downloads
Published
Issue
Section
License
Copyright (c) 2023 Mario Ávila-Gutierrez, Salvador Gutierrez-Portocarrero, Luis Corono-Elizarrarás, Mario A. Alpuche Aviles

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









