Comparison of the chemical composition and biological activities of essential oils from two Satureja species: Molecular docking studies
DOI:
https://doi.org/10.29356/jmcs.v67i1.1816Keywords:
S. khuzistanica, S. rechingeri, anticholinesterase activity, molecular docking, anticancer activityAbstract
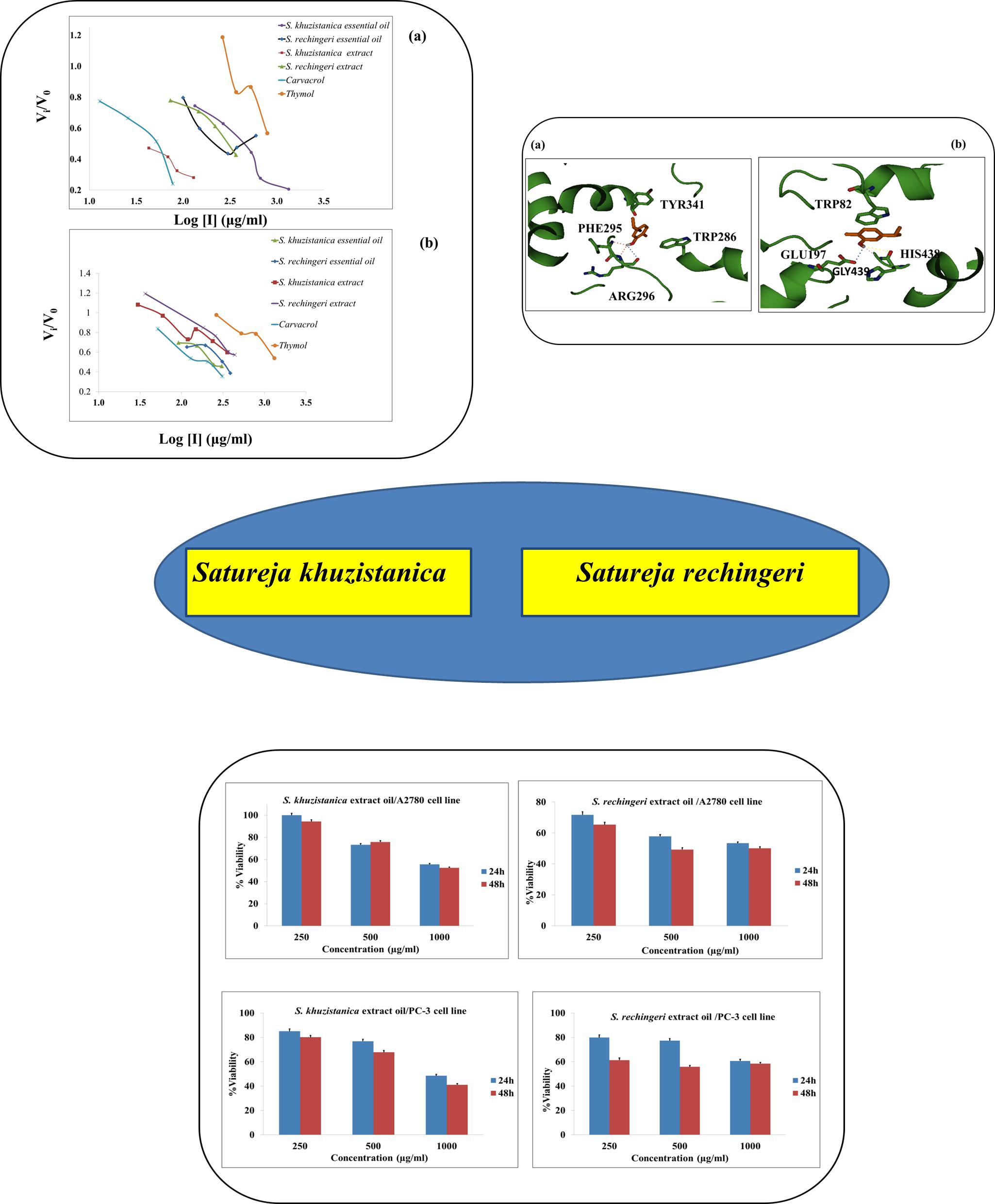
The Satureja species (family Lamiaceae) are economically important plants; they have been used as medicinal plants, flavoring in food, and cosmetic material for centuries. The volatile oils of two Satureja species, S. khuzistanica and S. rechingeri, were obtained by hydrodistillation method with Clevenger-type apparatus. The chemical composition of oils was analyzed by gas chromatography coupled with mass spectrometry (GC-MS). The major constituent of S. khuzistanica oil was Carvacrol (68.7%) and those of S. rechingeri oil were Thymol (51.28%) and Carvacrol (22.08%). Anticholinesterase and anticancer activities were screened by Ellman’s method and MTT assay, respectively. Besides, the role of non-covalent interactions in cholinesterase enzyme (ChE) inhibition by the main ingredient, Carvacrol, was studied through docking calculations. The inhibitory activity of S. khuzistanica oil was higher than those of S. rechingeri oil with IC50: 377.14±2.36 and 251.37±1.88 µg/ml against acetylcholinesterase enzyme (AChE) and butyrylcholinesterase enzyme (BChE). S. rechingeri essential oil was found to possess relatively moderate cytotoxic activity with IC50 values of 488.96±3.19 µg/ml and 767.22±3.19 µg/ml on A2780 and PC-3 cells, respectively. The role of hydrogen bonding and π…π stacking interactions in enzyme inhibition by a common ingredient, Carvacrol, was characterized.
Resumen. Las especies Satureja (familia Lamiaceae) son plantas económicamente relevantes; durante siglos se han utilizado como plantas medicinales, saborizantes en alimentos y material cosmético. Se obtuvieron los aceites volátiles de dos especies de Satureja, S. khuzistanica y S. rechingeri, empleando el método de hidrodestilación con un aparato tipo Clevenger. La composición química de los aceites se analizó mediante cromatografía de gases acoplada a espectrometría de masas (GC-MS). El componente principal del aceite de S. khuzistanica fue el carvacrol (68,7 %) y los del aceite de S. rechingeri fueron el timol (51,28 %) y el carvacrol (22,08 %). Se evaluó la actividad anticolinesterasa y anticancerígena emplenado el método de Ellman y el ensayo MTT, respectivamente. Además, se estudió el papel de las interacciones no covalentes en la inhibición de la enzima colinesterasa (ChE) por parte del ingrediente principal, Carvacrol, mediante cálculos de acoplamiento. La actividad inhibidora del aceite de S. khuzistanica fue superior a la del aceite de S. rechingeri con IC50: 377,14±2,36 y 251,37±1,88 µg/ml frente a la enzima acetilcolinesterasa (AChE) y la enzima butirilcolinesterasa (BChE). Se encontró que el aceite esencial de S. rechingeri posee una actividad citotóxica relativamente moderada con valores IC50 de 488,96±3,19 µg/ml y 767,22±3,19 µg/ml en células A2780 y PC-3, respectivamente. Se caracterizó el papel de los enlaces de hidrógeno y las interacciones de apilamiento π…π en la inhibición enzimática por el Carvacrol.
Downloads
References
Bachurin, S.O.; Bovina, E.V.; Ustyugov, A.A. Med. Res. Rev. 2017, 37, 1186-1225. DOI: https://doi.org/10.1002/med.21434
Klimova, B.; Kuca, K. J. Appl. Biomed. 2015, 3, 257-261. DOI: https://doi.org/10.1016/j.jab.2015.07.004
Klafki, H-W.; Staufenbiel M.; Kornhuber, J.; Wiltfang, J. Brain. 2006, 129, 2840-2855. DOI: https://doi.org/10.1093/brain/awl280
Nakhjiri, M.; Safavi, M.; Alipour, E.; Emami, S. Eur. J. Med. Chem. 2012, 50, 113-123. DOI: https://doi.org/10.1016/j.ejmech.2012.01.045
Nabavi, M.; Esmaeilzadeh, H.; Arshi, S.; Fallahpour, M.; Rezaei, N. Allergol Immunopathol. (Madr). 2014, 42, 85-7. DOI: https://doi.org/10.1016/j.aller.2012.10.003
Newman, D.J.; Cragg, G.M. J. Nat. Prod. 2020, 83, 770-803. DOI: https://doi.org/10.1021/acs.jnatprod.9b01285
Al-Rimawi, F.; Jaradat, N.; Qneibi, M.; Hawash, M.; Emwas, N. Eur. J. Integr. Med. 2020, 38, 101196. DOI: https://doi.org/10.1016/j.eujim.2020.101196
Lane, R.M.; Potkin, S.G.; Enz, A. Int. J. Neuropsychopharmacol. 2006, 9, 101-124. DOI: https://doi.org/10.1017/S1461145705005833
Karakaya, S.; Özdemir, Ö; Koka, M.; Demirci, B; Aksakal, Ö; Turkez, H; Baser, K. H.C. J. Essent. Oil Res. 2020, 32, 436-448. DOI: https://doi.org/10.1080/10412905.2020.1787884
Dirtinova, L.; Dobes, P.; Pohanka, M. J. Appl. Biomed. 2014, 12, 285-290. DOI: https://doi.org/10.1016/j.jab.2014.01.010
Wilkinson, D.G.; Francis, P.T.; Schwam, E.; Payne-Parrish, J. Drug Aging. 2004, 21, 453-478. DOI: https://doi.org/10.2165/00002512-200421070-00004
Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh D. Eur. J. Med. Chem. 2013, 70, 165-188. DOI: https://doi.org/10.1016/j.ejmech.2013.09.050
Inglis, F. Int. J. Clin. Pract. Suppl. 2002, 127, 45-63.
Saab, A.M.; Lampronti, I.; Borgatti, M.; Finotti, A.; Harb, F.; Safi, S.; Gambari, R. Nat. Prod. Res. 2012, 26, 2227-2231. DOI: https://doi.org/10.1080/14786419.2011.643885
Ashour, H. M. Cancer. Biol. Ther. 2008, 7, 399-403. DOI: https://doi.org/10.4161/cbt.7.3.5367
Jeune, M.A.L.; Kumi-Diaka, J.; Brown, J. J. Med. Food. 2005, 8, 469 - 475. DOI: https://doi.org/10.1089/jmf.2005.8.469
Gezici, S. Ann. Phytomed. 2018, 7, 38-45. DOI: https://doi.org/10.21276/ap.2018.7.2.5
Sonboli, A.; Fakhari, A.R.; Kanani, M.R.; Yousefzadi, M. Z. Naturforsch C. 2004, 59, 777-781. DOI: https://doi.org/10.1515/znc-2004-11-1202
Abad, M.J.; Bermejo, P.; Gonzales, E.; Iglesias, I.; Irurzun, A.; Carrasco, L. Gene Pharmacol. 1999, 32, 499-503. DOI: https://doi.org/10.1016/S0306-3623(98)00214-6
Hajhashemi, V.; Ghannadi, A.; Pezeshkian, S.K. J. Ethnopharmacol. 2002, 82, 83-87. DOI: https://doi.org/10.1016/S0378-8741(02)00137-X
Cetojevic-simin, D.D.; Canadanovic-Brunet, J.M.; Bogdanovic, G.M.; Cetkovic, G.S.; Tumbas, V.T.; S.M. Djilas. J. Buon. 2004, 9, 443-449.
Mozafarian, V. Lexicon of Iranian plant names. Publishing contemporary vocabulary. Tehran Press, Tehran, Iran, 1996, 234-251.
Rechinger, K.H. Satureja. In: Rechinger KH and Hedge IC (Eds), Flora Iranica: Labiatae. Akademische Druck und Verlagsanstalt, Graz, Austria, 1982, 150, 495-504.
Jamzad, Z. Iran. J. Bot. 1994, 6, 215-218.
Jamzad, Z. Satureja rechingeri (Labiatae) - a new species from Iran, Annalen des Naturhistorischen Museums in Wien. Serie B für Botanik und Zoologie, 98. Bd., Suppl. Festschrift 90 Jahre Karl Heinz Rechinger. 1996, 75-77.
Alizadeh, A. Z. Naturforsch. 2015, 70, 51-58. DOI: https://doi.org/10.1515/znc-2014-4121
Abdollahi, M.; Salehnia, A.; Mortazavi, S.H.; Ebrahimi, M.; Shafiee, A.; Fouladian, F.; Keshavarz, K.; Sorouri, S.; Khorasani, R.; Kazemi, A. Med. Sci. Monit. 2003, 9, BR331-335.
Amanlou, M.; Fazeli, M.R.; Arvin, A.; Amin, H.G.; Farsam, H. Fitoterapia. 2004, 75, 768-70. DOI: https://doi.org/10.1016/j.fitote.2004.09.005
Yousefzadi, M.; Riahi-Madvar, A.; Hadian, J.; Rezaee, F.; Rafiee, R.; Biniaz, M. J. Immunotoxicol. 2014, 11, 50-55. DOI: https://doi.org/10.3109/1547691X.2013.789939
Amanlou, M.; Farsam, H.; Babaei, N; Saheb Jamei, M.; Tohi Dast Akrad, Z.; Salehnia, A. Daru. J. Pharm. Sci. 2007, 15, 231-235.
Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. Biochem. Pharmacol. 1961, 7, 88-90. DOI: https://doi.org/10.1016/0006-2952(61)90145-9
Trott, O.; Olson, A.J. J. Comput. Chem. 2010, 31, 455-461. DOI: https://doi.org/10.1002/jcc.21334
Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. J. Comput. Chem. 1998, 19, 1639 - 1662. DOI: https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
Weiner, S.J.; Kollman, P.A.; Case, D.A.; Singh, U.C.; Ghio, C.E.; Alagona, G.; Profeta, S.; Weiner, P. J. Am. Chem. Soc. 1984, 106, 765-784. DOI: https://doi.org/10.1021/ja00315a051
Farsam, H.; Amanlou, M.; Radpour, M.R.; Salehinia, A.N.; Shafiee, A. Flavour. Fragr. J. 2004, 19, 308-310. DOI: https://doi.org/10.1002/ffj.1300
Hadian, J.; Esmaeili, H.; Nadjafi, F.; Khadivi-Khubb, A. Ind. Crops. Prod. 2014, 61, 403-409. DOI: https://doi.org/10.1016/j.indcrop.2014.07.034
Sefidkon, F.; Abbasi, K.; Jamzad, Z.; Ahmadi, S. Food Chemistry. 2007, 100, 1054-1058. DOI: https://doi.org/10.1016/j.foodchem.2005.11.016
Farzaneh, M.; Kiani, H.; Sharifi, R.; Reisid, M.; Hadian, J. Postharvest. Biol. Technol. 2015, 109, 145-151. DOI: https://doi.org/10.1016/j.postharvbio.2015.06.014
Taban, A.; Saharkhiz, M.J.; Hadian, J. Biol. Agric. Hortic. 2013, 29, 244-257. DOI: https://doi.org/10.1080/01448765.2013.830275
Nooshkam, A.; Mumivand, H.; Hadian, J.; Alemardan, A.; Morshedloo, M.R. J. Appl. Res. Med. Aromat. Plants. 2017, 6, 126-130. DOI: https://doi.org/10.1016/j.jarmap.2017.04.002
Ozadali-Sari, K.; Küçükkılınç, T.T.; Ayazgok, B.; Balkan, A.; Unsal-Tan, O. Bioorg. Chem. 2017, 72, 208-214. DOI: https://doi.org/10.1016/j.bioorg.2017.04.018
Chaiyana, W.; Okonogi, S. Phytomedicine. 2012, 19, 836-839. DOI: https://doi.org/10.1016/j.phymed.2012.03.010
Kivrak, I.; Emin Duru, M.; Ozturk, M.; Mercan, N.; Harmandar, M.; Topçu, G. Food Chem. 2009, 116, 470-479. DOI: https://doi.org/10.1016/j.foodchem.2009.02.069
Belkacem, N.; Khettal, B.; Hudaib, M.; Bustanji, Y.; Abu-Irmaileh, B.; Amrine, C.S.M. Eur. J. Integr. Med. 2021, 42, 101292. DOI: https://doi.org/10.1016/j.eujim.2021.101292
Samaradivakara, S.P.; Samarasekera, R.; Handunnetti, S.M., Weerasena, O.V.D.S. J. Ind. Crops. Prod. 2016; 83: 227-234. DOI: https://doi.org/10.1016/j.indcrop.2015.12.047
Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. J. Nutr. 2003, 133, 1286-1290. DOI: https://doi.org/10.1093/jn/133.5.1286
Alok, S.; Kumar Jain, S.; Verma, A.; Kumar, M.; Mahor, A.; Sabharwal, M. Asian. Pac. J. Trop. Biomed. 2014, 4, 78-84. DOI: https://doi.org/10.1016/S2221-1691(14)60213-6
Marotti, M.; Dellacecca, V.; Piccaglia, R.; Giovanelli, E. Acta Horticulturae. 1993, 331, 63-70. DOI: https://doi.org/10.17660/ActaHortic.1993.331.9
Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M.; Milos, M. Phytother Res. 2007, 21, 259-261. DOI: https://doi.org/10.1002/ptr.2063
Bhalla, Y.; Gupta, V. K.; Jaitak, V. J. Sci. Food. Agric. 2013, 93, 3643 - 3653. DOI: https://doi.org/10.1002/jsfa.6267
Gautam, N.; Mantha, A.K.; Mittal, S. Biomed. Res. Int. 2014, 2014, 154106. DOI: https://doi.org/10.1155/2014/154106
Fitsiou, E.; Anestopoulos, I.; Chlichlia, K.; Galanis, A.; Kourkoutas, I.; Panayiotidis, M.I.; Pappa, A. Anticancer Res. 2016, 36, 5757-5764. DOI: https://doi.org/10.21873/anticanres.11159
Arcamone, F. Doxorubicin: Anticancer Antibiotics, Elsevier Science, 2012.
(a) Kang, S. H.; Kim, Y.S.; Kim, E.K.; Hwang, J-W.; Jeong, J-H.; Lee, J-W.; Moon, S-H.; Jeon, B-T.; Park, P-J. J. Microbiol. Biotechnol. 2016, 26, 28-37; (b) Arab, H.A.; Fathi, M.; Mortezai, E.; Hosseinimehr, S.J. Pharm. Biomed. Res. 2015, 1, 26-31; (c) Yeh, J.H.; Chou, C.T.; Chen, I.S.; Lu, T.; Lin, K-L.; Yu, C-C.; Liang, W-Z.; Chang, H-T.; Kuo, C-C.; Ho, C-M.; Chang, W-T.; Shieh, P.; Jan, C-R. Chin. J. Physiol. 2017, 60, 32-40.
(a) Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Anticancer Drugs. 2015, 26, 813-823; (b) Yin, Q.H.; Yan, F.X.; Zu, X.Y.; Wu, Y-h.; Wu, X-p.; Liao, M-c.; Deng, S-w.; Yin, L-l.; Zhuang, Y-Z. Cytotechnology. 2012, 64, 43-51; (c) Arunasree, K.M. Phytomedicine. 2010, 17, 581-588.
Koparal, A.T.; Zeytinoglu, M. Cytotechnology. 2003, 43, 149-154. DOI: https://doi.org/10.1023/B:CYTO.0000039917.60348.45
Burt, S. Int. J. Food Microbiol, 2004, 94, 223-253. DOI: https://doi.org/10.1016/j.ijfoodmicro.2004.03.022

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2023 Behrouz Ezatpour, Niloufar Dorosti, Elham Rezaee, Fatemeh Ghaziani

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








