Phytochemical Profile, Antioxidant and Antibacterial Activities of Artemisia absinthium L. Collected from Tunisian Regions
DOI:
https://doi.org/10.29356/jmcs.v66i3.1709Keywords:
Wormwood, Artemisia absinthium L., phenolic composition, essential oil composition, antioxidant activity, antimicrobial activity, regional factorAbstract
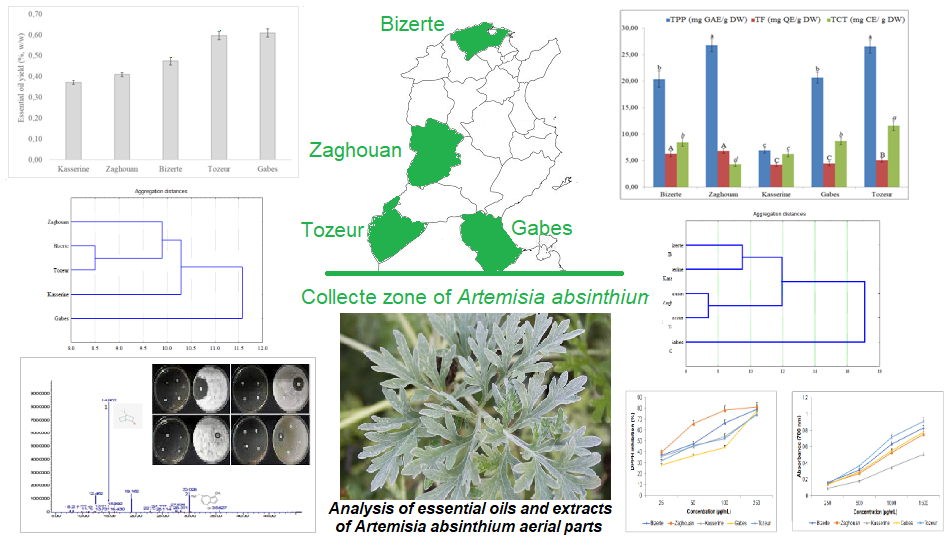
Abstract. The aim of this comparative research was to determine the chemical composition, antioxidant and antibacterial activities of the methanolic extracts and essential oils (EOs) of Artemisia absinthium aerial parts from five different regions (Bizerte, Zaghouan, Kasserine, Gabes and Tozeur). The polyphenol and flavonoid contents significantly varied (P < 0.05) among the studied regions with maximal contents observed in Zaghouan. Based on the High Performance Liquid Chromatography results, quercetin and isorhamnetin were the main compounds and their percentages were region dependent. The methanolic extract of Zaghouan showed the highest scavenging ability of DPPH (IC50 = 31.46 ± 1.42 µg/mL). A. absinthium EOs from of the different regions were found to interestingly inhibit the growth of both Gram-negative and Gram-positive bacteria strains. The antibacterial effect was strongly related to the organoleptic EO quality. The EO of Zaghouan exhibited an important inhibitory effect with an inhibition zone estimated at 31 mm against Escherichia coli strain. The EO composition was obtained by GC-MS analysis showing the presence of thirty-five compounds. Camphor (49.70 ± 2.34 %) and chamazulene (25.41 ± 0.61 %) were the main constituents. These results suggested that the north regions have a high potential for selecting varieties rich on bioactive volatile and phenolic compounds.
Resumen. El objetivo de esta investigación fue determinar y comparar la composición química, las actividades antioxidantes y antibacterianas de los extractos metanólicos y de los aceites esenciales (AE) de las partes aéreas de Artemisia absinthium de cinco regiones (Bizerta, Zaghouan, Kasserine, Gabes y Tozeur). Los contenidos de polifenoles y flavonoides variaron significativamente (P < 0,05) entre las regiones estudiadas con contenidos máximos observados en Zaghouan. De acuerdo con los resultados de la cromatografía líquida de alta resolución, la quercetina y la isorhamnetina fueron los compuestos principales y sus porcentajes dependieron de la región. El extracto metanólico de Zaghouan mostró la mayor capacidad secuestrante de DPPH (IC50 = 31.46 ± 1.42 µg mL-1). Se descubrió que los aceites esenciales de A. absinthium de las diferentes regiones inhibían de manera interesante el crecimiento de cepas de bacterias Gram-negativas y Gram-positivas. El efecto antibacteriano estuvo fuertemente relacionado con la calidad organoléptica del AE. El AE de Zaghouan exhibió un importante efecto inhibidor con un halo de inhibición estimado en 31 mm frente a una cepa de Escherichia coli. La composición de AE se obtuvo mediante análisis GC-MS y mostró la presencia de treinta y cinco compuestos. El alcanfor (49.70 ± 2.34 %) y el camazuleno (25.41 ± 0.61 %) fueron los principales constituyentes. Estos resultados sugirieron que las regiones del norte tienen un alto potencial para seleccionar variedades ricas en compuestos bioactivos volátiles y fenólicos.
Downloads
References
Abad, M. J.; Bedoya, L. M. ;Apaza, L. ;Bermejo, P. Molecules. 2012, 17, 2542-2566. DOI: https://doi.org/10.3390/molecules17032542
Ramakrishna, A.; Ravishankar, G. A. Plant Signal. Behav. 2011, 6, 1720-1731. DOI: https://doi.org/10.4161/psb.6.11.17613
Pavarini, D. P.; Pavarini, S. P.; Niehues, M. ; Lopes, N. P. Anim. Feed Sci. Technol. 2012, 176, 5-16. DOI: https://doi.org/10.1016/j.anifeedsci.2012.07.002
Szakiel, A.; Paczkowski, C.; Henry, M. Phytochem. Rev. 2011, 10, 471-491. DOI: https://doi.org/10.1007/s11101-010-9177-x
Stashenko, E. E.; Martínez, J. R.; Ruíz, C. A.; Arias, G.; Durán, C.; Salgar, W.; Cala M. J. Sep. Sci. 2010, 33, 93-103. DOI: https://doi.org/10.1002/jssc.200900452
Vilela, E. C.; Duarte, A. R.; Naves, R. V.; Santos, S. C.; Seraphin, J. C.; Ferri, P. H. J. Braz. Chem. Soc. 2013, 24, 873-879.
Telascrea, M.; de Araújo, C. C.; Marques, M. O. M.; Facanali, R.; Moraes, P.; Cavalheiro, A. J. Biochem. Syst. Ecol. 2007, 35, 222-232. DOI: https://doi.org/10.1016/j.bse.2006.09.015
Rahimmalek, M.; Tabatabaei, B. E. S.; Etemadi, N.; Goli, S. A. H.; Arzani, A.; Zeinali, H. Ind. Crop. Prod. 2009, 29, 348-355. DOI: https://doi.org/10.1016/j.indcrop.2008.07.001
Tan, R. X.; Zheng, W. F.; Tang, H. Q. Planta Med. 1998, 64, 295-302. DOI: https://doi.org/10.1055/s-2006-957438
Hayat, M. Q.; Khan, M. A.; Ashraf, M.; Jabeen, S. Ethnobot. Res. Appl. 2009, 7, 147-162. DOI: https://doi.org/10.17348/era.7.0.147-162
Wright, C. W. Taylor and Francis Inc, New York (NY), 2002.
Alan, A. D.; Calzada, F.;Cervantes, J. A.; Torres, J.; Ceballos, G. M. J. Ethnopharmacol. 2005, 100, 153-157. DOI: https://doi.org/10.1016/j.jep.2005.02.022
Caner, A.; Doskaya, M.; Degirmenci, A.; Can, H.; Baykan, S.; Uner, A.; Başdemir, G.; Zeybek, U.; Gürüz, Y. Exp. Parasitol. J. 2008, 119, 173-179. DOI: https://doi.org/10.1016/j.exppara.2008.01.012
Ramazani, A.; Sardari, S.; Zakeri, S.; Vaziri, B. Parasitol. Res. 2010, 107, 593-599. DOI: https://doi.org/10.1007/s00436-010-1900-4
Amat, N.; Upur, H.; Blazekovic, B. J Ethnopharmacol. 2010, 131, 478-484. DOI: https://doi.org/10.1016/j.jep.2010.07.023
Bora, K. S.; Sharma, A. J. Ethnopharmacol. 2010, 129, 403-409. DOI: https://doi.org/10.1016/j.jep.2010.04.030
Daradka, H. M.; Abas, M. M.; Mohammad, M. A. M.; Jaffar, M. M. Comp. Clin. Pathol. 2014, 23, 1733-1742. DOI: https://doi.org/10.1007/s00580-014-1963-1
Wake, G.; Court, J.; Pickering, A.; Lewis, R.; Wilkins, R.; Perry, E. J. Ethnopharmacol. 2000, 69, 105-114. DOI: https://doi.org/10.1016/S0378-8741(99)00113-0
Guarrera, P. M. Fitoter. 2005, 76, 1-25. DOI: https://doi.org/10.1016/S0242-6498(05)80108-7
Benkhaled, A.; Boudjelal, A.; Napoli, E.; Baali, F.; Ruberto, G. Asian Pac. J. Trop. Biomed. 2020, 10, 496-504. DOI: https://doi.org/10.4103/2221-1691.294089
Hoffmann, D. Medical herbalism: the science and practice of herbal medicine. Healing Arts Press, 2003, 246-253.
Scott, T. L.; Buhner, S. H. Heal. Art. Press. 2010.
Canadanovic-Brunet, J. M.; Djilas, S. M.; Cetkovic, G. S.; Tumbas, V. T. J. Sci. Food Agric. 2005, 85, 265-272 DOI: https://doi.org/10.1002/jsfa.1950
Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj, A. J. Chem. 2015, 2015, 1-12. DOI: https://doi.org/10.1155/2015/804658
Riahi, L.; Ghazghazi, H.; Ayari, B.; Aouadhi, C.; Klay, I.; Chograni, H.; Cherif, A.; Zoghlami, N. Ind. Crop. Prod. 2015, 66, 96-102. DOI: https://doi.org/10.1016/j.indcrop.2014.12.036
Pazhouh, H. K.; Hamedi, S.; Hosseini, S. M. R.; Taghipour, A.; Javadi, B.; Noras, M. Tradit. Med. Res. 2020, 5, 498-506. DOI: https://doi.org/10.53388/TMR20200210160
Kaoudoune, C.; Benchikh, F.; Benabdallah, H.; Loucif, K.; Mehlous, S.; Amira, S. JDDT. 2020, 10, 153-156. DOI: https://doi.org/10.22270/jddt.v10i4.4253
Rezaeinodehi, A.; Khangholi, S. Pak. J. Biol. Sci. 2008, 11, 946-949. DOI: https://doi.org/10.3923/pjbs.2008.946.949
Derwich, E.; Benziane, Z.; Boukir, A. Elect. J. Envir. Agric. Food Chem. 2009, 8, 1202-1211.
Martín, L.; Mainar, A. M.; González-Coloma, A.; Burillo, J.; Urieta, J. S. J. Supercrit. Fluids. 2011, 56, 64-71. DOI: https://doi.org/10.1016/j.supflu.2010.11.017
Adams, R. P. 3rd edition, Carol Stream, Illinois, USA: Allured Publishing Corporation, 2004.
Sibanda, S.; Chigwada, G.; Poole, M.; Gwebu, E. T.; Noletto, J. A.; Schmidt, J. M.; Rea, A. I.; Setzer, W. N. J. Ethnopharmacol. 2004, 92, 107-111. DOI: https://doi.org/10.1016/j.jep.2004.02.010
Mau, J. L.; Chao, G. R.; Wu, K. T. J. Agric. Food Chem. 2001, 49, 5461-5467. DOI: https://doi.org/10.1021/jf010637h
Dewanto, V.; Wu, X.; Adom, K. K.; Liu, R. H. J. Agric. Food Chem. 2002, 50, 3010-3014. DOI: https://doi.org/10.1021/jf0115589
Sun, B.; Richardo-da-Silvia, M.; Spranger, I. J. Agric. Food Chem. 1998, 46, 4267-4274. DOI: https://doi.org/10.1021/jf980366j
Prieto, P.; Pineda, M.; Aguilar, M. Anal. Biochem. 1999, 269, 337-341. DOI: https://doi.org/10.1006/abio.1999.4019
Hanato, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Chem. Pharmaceut. Bull. 1988, 36, 2090-2097. DOI: https://doi.org/10.1248/cpb.36.2090
Oyaizu, M. Jap. J. Nutr. 1986, 44, 307-315. DOI: https://doi.org/10.5264/eiyogakuzashi.44.307
Bagamboula, M.; Uyttendaele, J.; Debevere, M. Food Microbiol. 2003, 21, 33-42. DOI: https://doi.org/10.1016/S0740-0020(03)00046-7
Lopez-Lutz, D.; Alviano, D. S.; Alviano, C. S.; Kolodziejczyk, P. P. Phytochem. 2008, 69, 1732-1738. DOI: https://doi.org/10.1016/j.phytochem.2008.02.014
Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A., Yildirin, A. J. Agric. Food Chem. 2005, 53, 9452-9458. DOI: https://doi.org/10.1021/jf0516538
Vieira, T. M.; Dias, H. J.; Medeiros, T. C. T.; Grundmann, C. O.; Groppo, M.; Heleno, V. C. G.; Martins, C. H. G.; Cunha W. R.; Crotti A. E. M.; Silva E. O. J. Essent. Oil-Bear. Pl. 2017, 20, 123-131. DOI: https://doi.org/10.1080/0972060X.2016.1257370
Orav, A.; Raal, A.; Arak, E.; Müürisepp, M.; Kailas, T. Proceed. Eston. Acad. Sci. Chem. 2006, 55, 155-165. DOI: https://doi.org/10.3176/chem.2006.3.04
Safayhi, H.; Sabieraj, J.; Sailer, E. R.; Ammon, H. P. T. Planta Med. 1994, 60, 410-413. DOI: https://doi.org/10.1055/s-2006-959520
Mahmoudi, M.; Ebrahimzadeh, M. A.; Ansaroudi, F.; Nabavi, S. F.; Nabavi, S. M. Afr. J. Biotechnol. 2009, 8, 7170-7175.
Zengin, G.; Mollica, A.; Aktumsek, A.; Picot, C. M. N.; Mahomoodally, M. F. Eur. J. Integr. Med. 2017, 12, 135-141. DOI: https://doi.org/10.1016/j.eujim.2017.05.010
Sidaoui, F.; Belghith, I. S.; Yemmen, M.; Mraihi, F.; Barth, D.; Trabelsi-Ayadi, M.; Cherif, J.K. Int. J. Pharmaceut. Clin. Res. 2016, 8, 1178-1185.
David, A. V. A.; Arulmoli, R.; Parasuraman, S. Pharmacogn. Rev. 2016, 10, 84-89. DOI: https://doi.org/10.4103/0973-7847.194044
Kim, J. E.; Lee, D. E.; Lee, K. W.; Son, J. E.; Seo, S. K.; Li, J.; Jung S. K.; Heo Y. S.; Mottamal M.; Bode A. M.; Dong Z.; Lee H. J. Can Prev Res. 2011, 4, 582–591. DOI: https://doi.org/10.1158/1940-6207.CAPR-11-0032
Lee Y. J.; Thiruvengadam, M.; Chung, I. M.; Nagella P. Aust. J. Crop. Sci. 2013, 7, 1921-1926.
Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Chem. Cent. J. 2012, 6, 1-12. DOI: https://doi.org/10.1186/1752-153X-6-97
Kiokias, S.; Varzakas, T. Food Chem. 2014, 150, 280-286. DOI: https://doi.org/10.1016/j.foodchem.2013.10.112
Bubols, G. B.; Vianna, D. R.; Medina-Remónd, A.; Poser, G.; Lamuela-Raventos, R. M.; Eifler-Lima, V. L.; Garcia, S. C. Med. Chem. 2013, 13, 318-334. DOI: https://doi.org/10.2174/1389557511313030002
Moghaddam, P. Z.; Kamali, H.; Imani, M.; Mohammadi, A. J. Med. Pl. Nat. Prod. 2016, 1, 1-9.
Juteau, F.; Jerkovic, I.; Masotti, V.; Milos, M.; Mastelic, J.; Bessière, J. M.; Viano, J. Planta Med. 2003, 69, 158-161. DOI: https://doi.org/10.1055/s-2003-37714
Kheyar, N.; Meridja, D.; Belhamel, K. Alg. J. Nat. Prod. 2014, 2, 18-26.
Saada, M.; Falleh, H.; Jalleli, I.; Snoussi, M.; Ksouri, R. S. Afr. J. Bot. 2014, 94, 114-121.

Downloads
Published
Issue
Section
License
Copyright (c) 2022 Wissem Aidi Wannes

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









