Phytochemistry, Mineral Estimation, Nutritional, and the In Vitro Anti-Sickling Potentials of Oil Extracted from the Seeds of Mucuna Flagellipes
DOI:
https://doi.org/10.29356/jmcs.v68i2.1898Keywords:
Anti-sickling, Mucuna flagellipes, Zinc, Vitamins, unsaturated fats, PolymerizationAbstract
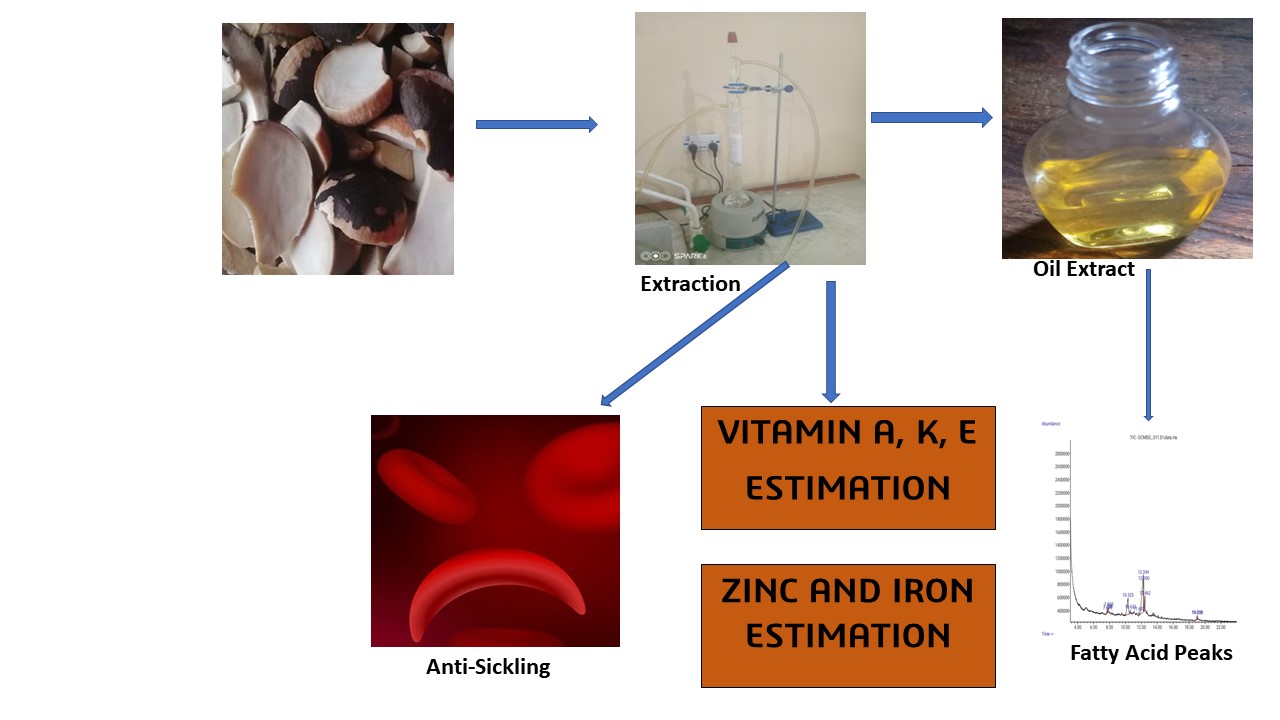
Abstract. Sickle cell disease is an inherited blood disorder indicative of red blood cells becoming sickle-shaped. The study investigated the in vitro anti-sickling potentials of the seed oil of M. flagellipes. The phytochemistry (fatty acids, vitamins, and minerals) was also determined using standard protocols. Finally, nutritional calculations on the oil were performed to determine its suitability for nutritional purposes. The result showed high zinc content (780 ± 2.50 µg/ 100 mg), while low iron content was observed (170 ± 1.30 µg/ 100 mg). Vitamin analysis showed the presence of vitamins A, E and K with values of 220 ± 1.60, 370 ± 2.20, and 197 ± 0.23 µg/100 mg respectively. The fatty acid profile revealed oleic (31.87 %) and linoleic (18.30 %) fatty acids as the major unsaturated fat in the oil, while palmitic fatty acid (5.91 %) was the major saturated fatty acid. Nutritional calculations showed high PUFA/SFA (2.07), MUFA/SFA (3.62), and UI (68.47). However, the index of thrombogenicity (0.07) and atherogenicity (0.11) was low in the seed oil. Finally, the in vitro anti-sickling potentials of the seed oil showed the oil inhibited and reversed sickling in a dose-dependent manner. Hbs polymerization was also inhibited and Fe2+/Fe3+ was upregulated following treatment with the seed oil. Collectively, the oil showed good anti-sickling potentials, which can be labelled to the presence of zinc, vitamins and unsaturated fat. The nutritional calculations suggest that the seed oil is cardio-friendly and does not pose any nutritional threat
Resumen. La anemia falciforme es un trastorno hereditario de la sangre que indica que los glóbulos rojos adquieren forma de hoz. Este estudio investigó los potenciales de evitar la malformación de células falciformes (anti-sickling) in vitro por el aceite de la semilla de Macuna. flagellipes. La fitoquímica (ácidos grasos, vitaminas y minerales) se determinó utilizando protocolos estándar. Finalmente, se realizaron cálculos nutricionales del aceite para determinar su idoneidad para fines nutricionales. El resultado mostró alto contenido de zinc (780 2,50 µg/100mg), mientras que se observó bajo contenido de hierro (170 1,30 µg/100mg). El análisis vitamínico mostró presencia de vitamina A, E y K con valores de 220 ± 1.60, 370 ± 2.20 y 197 ± 0.23 µg/100 mg, respectivamente. El perfil de ácidos grasos reveló ácidos oleicos (31.87 %) y linoleico (18.30 %) como las principales grasas insaturadas del aceite, mientras que el ácido palmítico (5.91 %) fue el principal ácido graso saturado. Los cálculos nutricionales mostraron un alto PUFA/SFA (2.07), MUFA/SFA (3.62), UI (68.47). Sin embargo, el índice de trombogenicidad (0.07) y aterogénesis (0.11) fue bajo en el aceite de semilla. Por último, los potenciales para evitar la malformación de células falciformes in vitro por aceite de semilla mostraron que el aceite inhibió y revirtió la enfermedad de una manera dependiente de la dosis. La polimerización de Hbs también fue inhibida y Fe2+/Fe3+ fue sobreregulada después del tratamiento con el aceite de la semilla. Colectivamente, el aceite mostró un buen potencial evitar la malformación de células falciformes, que puede ser atribuido a la presencia de zinc, vitaminas y grasa insaturada. Los cálculos nutricionales sugieren que el aceite de semilla es cardio-amigable y no representa ninguna amenaza nutricional.
Downloads
References
Strouse, J. Handbook of Clin. Neurol. 2016, 138, 311-324. DOI: https://doi.org/10.1016/B978-0-12-802973-2.00018-5
Yenon, A. A.; Sangare, B.; Ndraman, D.; Sawadogo, D.; Yapi, H. F.; Nguessan, J. D.; Djaman, A. J. The Pharma. Chem. J. 2016, 3, 351-358.
Mpiana, P. T.; Mudogu, V.; Tshibagu, D. S. T.; Kitwa, E. K.; Kanagila, A. B.; Lumbu, J. B. S.; Ngbolua, K. N.; Atibu, E. K.; Kakuli, M. K. J. Ethnopharmacol. 2008, 120, I413-418. DOI: https://doi.org/10.1016/j.jep.2008.09.012
Mohammed, R. H.; Sulaiman, S. K. Int. J. Appl. Sci. Res. 2021, 4, 1-12.
Okpala, I.; Ibegbulam, O.; Duru, A.; Ocheni, S.; Emodi, I.; Ikefuna, A.; Umar, G.; Asinobi, I.; Madu, A.; Okoye, A.; Nwagha, T.; Oguonu, U.; Uamai, I.; Agwu, O.; Nonyelu, C.; Anike, U.; Agu, K.; Anigbo, C.; Chukwura, A.; Ugwu, O.; Herrada, S. Acta Pathologica, Microbiologica, et Immunologica Scandinavica 2011, 119, 442–448. DOI: https://doi.org/10.1111/j.1600-0463.2011.02751.x
Puppalwar, P. V.; Adole, P.; Dhok, A.; Bhatkulkar, P. Int. J. Therapeutic Appl. 2015, 25, 1-6
Pullar, J. M.; Carr, A. C.; Vissers, M. C. M. Nutrients 2018, 9, 866. DOI: https://doi.org/10.3390/nu9080866
Datta, D.; Namazzi, R.; Conroy, A. L.; Cusick, S. E.; Hume, H. A.; Tagoola, A.; Ware, R. E.; Opoka, R. O.; John, C. C. Trials 2019, 20, 460. DOI: https://doi.org/10.1186/s13063-019-3569-z
Khan, S.; Damanhouri, G.; Ahmed, T. J.; Halawani, S.; Ali, A.; Makki, A.; Khan, S. J. King Saud Univ. – Sci. 2022, 34. DOI: https://doi.org/10.1016/j.jksus.2022.101942
Ibrahim, N. K.; Ahmed, J. H.; Hassan, M. K. Singapore Med. J. 2010, 51, 230-234.
Moody, J. O.; Ojo, O. O.; Omotade, O. O.; Adeyemo, A. A.; Olumese, P. E.; Ogundipe, O. O. Phytother. Res. 2003, 17, 1173–1176. DOI: https://doi.org/10.1002/ptr.1323
Nwokocha, L. M; Williams, P. A. Food Hydrocolloids 2009, 23, 1394-1397. DOI: https://doi.org/10.1016/j.foodhyd.2008.08.014
Ihedioha, J. N.; Okoye, C. O. Am. J. of Plant Nutr. Fertilization Tech. 2011, 1, 55-63. DOI: https://doi.org/10.3923/ajpnft.2011.55.63
Okwu, D. E.; Okoro, E. Med. Aromatic Plant Sci. Biotech. 2007, 1, 103- 106. DOI: https://doi.org/10.1002/app.26560
Uchegbu, R. I.; Ngozi-Olehi, L. C.; Mbadiugha, C. N.; Ahuchogu, A. A., Ogbuagu, O. E. J. Nat. Sci. Res. 2015, 5, 12.
Ajayi, I. A.; Oderinde, R. A.; Kajogbola, D.; Uponi, J. I. Food Chemistry, 2006, 99, 1, 115-120. DOI: https://doi.org/10.1016/j.foodchem.2005.06.045
Abireh, I.; Ozioko, O.; Ikemefuna, O. J. Exp. Res. 2020, 8, 1. DOI: https://doi.org/10.9734/ajarr/2020/v14i430336
Jovita, E. E.; Ani, C. O.; Uzoma, I. C.; Pamela, A. S.; Onwuka, C. K.; Ayowumi, A. M.; Daniel, N. C. Afr. J. Pharmacy Pharmacol. 2016, 11, 582- 592.
Djote W. N.; Kotue, T. C.; Mafogang, B.; Ngo, S. F.; Pieme, A. C. J. Nutr. Food Lipid Sci. 2020, 1: 86-93.
Daak, A. A.; Lopez-Toledano, M. A.; Heeney, M. M. Compl. Therapies in Med. 2020, 52, 102482. DOI: https://doi.org/10.1016/j.ctim.2020.102482
Chukwu, C. N.; Onyedikachi, U. B.; Ejiofor, E. CMU J. Nat. Sci. 2022, 21, e2022010.
Ariyike, O. A.; Hezekiah, A. G.; Obajimi, A. O.; Olusola, O. O. GSC Biol. Pharmaceut. Sci. 2019, 7, 77–92.
Onyedikachi, U. B., Awah, F. M.; Chukwu, C. N.; Ejiofor, E. Acta Universitatis Cibiniensis. Series E: Food Tech. 2021, 25, 1-14. DOI: https://doi.org/10.2478/aucft-2021-0001
Onyedikachi, B.; Ejiofor, E.; Njoku, C.; Ejiofor, M.; Kanu, M. J. Mex. Chem. Soc. 2022, 66, 433- 443
De’ Leenheer, A. P.; Nelis, H. J.; Lambert, W. E.; Bauwens, R. M. J. Chromatography A 1988, 29, 13-58.
Gul, W.; Anwar, Z.; Qadeer, K.; Perveen, S.; Ahmad, I. J. Pharmacy Pharmaceut. Sci. 2015, 3, 14-22
AOAC, Official method of Analysis. 2005, 18th Edition 935.14 and 992.24.
Chen, J.; Liu, H. Int. J. Mol. Sci. 2020, 21, 5695. DOI: https://doi.org/10.3390/ijms21165695
Eke, R.; Ejiofor, E.; Oyedemi, S.; Onoja, S.; Omeh, N. J. Food Biochem. 2021, e13763.
McCormick, R. L.; Ratcliff, M.; Moens, L.; Lawrence, R. Fuel Processing Tech. 2007, 88, 651–657. DOI: https://doi.org/10.1016/j.fuproc.2007.01.006
Egba, S. I.; Emmanuel, N. T.; Ogugua, N. V.; Ndohnui, N. N. Int. J. Biochem. Biotech. 2012, 1, 226–229.
Ekeke, G. I.; Uwakwe, A. A.; Nwaoguikpe, R. N. Nig. J. Biochem. Mol. Biol. 2000, 16, 45–47.
Noguchi, C. T.; Schechter, A. N. Annual Rev. 1985, 4:239-245. DOI: https://doi.org/10.1146/annurev.bb.14.060185.001323
Tietz, N. W. Fundamentals of clinical chemistry, 2nd edn. W. B. Saunders Company, Philadelphia, 1976, 34-40.
Chikezie, P. C.; Uwakwe, A. A. Pharmacognosy Mag. 2011, 7, 121-125. DOI: https://doi.org/10.4103/0973-1296.80669
Fratianni, F.; d'Acierno, A.; Ombra, M. N.; Amato, G.; De Feo, V.; Ayala-Zavala, J. F.; Coppola, R.; Nazzaro, F. Frontiers in Nutr. 2021, 8, 775751. DOI: https://doi.org/10.3389/fnut.2021.775751
Hebbel, R. P.; Eaton, J. W.; Balasingam, M.; Steinber, M. H. J. Clin. Investig. 1982, 70, 1253-1259. DOI: https://doi.org/10.1172/JCI110724
Nwajagu, I. U.; Garba, A.; Nzelibe, H. C.; Chukwuekezie, N. E.; Abah, C. R.; Umar, A. T.; Anarado, C. S.; Kahu, J. C.; Olagunju, A.; Oladejo, A. A.; Bashiru I. Am. J. Food and Nutr. 2021, 9, 49-59.
Ray, D.; Deshmukh, P.; Goswami, K.; Garg, N. The Nat. Med. J. India, 2007, 20, 11–13.
Obi, C. D.; Okoye, J. I. Int. J. Innovative Food, Nutr. Sustainable Agric. 2017, 5, 18-24.
Oyedemi, S. O.; Eze, K.; Aiyegoro, A. O.; Ibeh, R. C., Ikechukwu, G. C., Swain, S. S.; Ejiofor, E.; Oyedemi, B.O. J. Biomolecular Struct. Dynamics 2021, 40: 9948- 9961. DOI: https://doi.org/10.1080/07391102.2021.1938228
Ejiofor, E. U.; Oyedemi, S. O.; Onoja, S. O.; SO, Omeh, N. Y. South Afr. J. Bot. 2022, 146, 213-221. DOI: https://doi.org/10.1016/j.sajb.2021.10.029
Temiye, E. O.; Duke, E. S.; Owolabi, M. A.; Renner, J. K. Anemia, 2011, 698586, 7. DOI: https://doi.org/10.1155/2011/698586
Miranda, C. T.; Vermeulen-Serpa, K. M.; Pedro, A. C.; Brandão-Neto, J.; Vale, S. H.; Figueiredo, M. S. J. Trace Elements in Med. Biol. 2022, 72, 126980. DOI: https://doi.org/10.1016/j.jtemb.2022.126980
Coates, T. D.; Wood, J. C. British. J. Haematology, 2017, 177, 703 -716. DOI: https://doi.org/10.1111/bjh.14575
Sani, M. A.; Adewuyi, J. O.; Babatunde, A. S.; Olawumi, H. O.; Shittu, R. O. Advances in Hematology, 2015, 386451, 5. DOI: https://doi.org/10.1155/2015/386451
Rees, D. C.; Williams, T. N.; Gladwin, M. T. Lancet, 2010, 376, 2018–2031. DOI: https://doi.org/10.1016/S0140-6736(10)61029-X
Dash, S.; Brewer, G.; Oelshlegel, F. Nature 1974, 250, 251–25. DOI: https://doi.org/10.1038/250251a0
Eaton, J. W.; Skelton, T. D., Swofford, H. S.; Kolpin, C. E.; Jacob, H. S. Nature, 1973, 246, 105. DOI: https://doi.org/10.1038/246105a0
Bao, B.; Prasad, A. S.; Beck, F. W. Translational Res. 2008, 152, 67–80. DOI: https://doi.org/10.1016/j.trsl.2008.06.001
Natta, C. L.; Tatum, V. L.; Chow, C. K. Annals New York Acad. Sci. 1992, 669, 365-367. DOI: https://doi.org/10.1111/j.1749-6632.1992.tb17125.x
Rifkind, J. M.; Heim, J. M. Biochem. 1997, 16, 4438–4443. DOI: https://doi.org/10.1021/bi00639a017
Monod, J.; Wyman, J.; Changeux, J. P. J. Mol. Biol. 1965, 12, 88-118. DOI: https://doi.org/10.1016/S0022-2836(65)80285-6
Eaton, W. A.; Henry, E. R., Hofrichter, J.; Mozzarelli, A. Nat. Struct. Biol. 1999, 6, 351-358. DOI: https://doi.org/10.1038/7586
Eaton, W. A.; Bunn, H. F. Blood 2017, 12, 2719–2726. DOI: https://doi.org/10.1182/blood-2017-02-765891
Dennis, D.; Roberts, A. in: Trease and Evans Pharmacognosy, The Alden Press, Oxford, Great Britain. 1990, 832.
Murray, R. K.; Granner, D. K.; Mayes, P. A.; Rodwell, V. W. in: Harper’s Illustrated Biochemistry, 27th edn. McGraw Hill Companies, New York. 2006
Osuagwu, C. G. Nig. J. Biochem. Mol. Biol. 2010, 25, 68–71.
Szpunar-Krok, E.; Wondołowska-Grabowska, A. Foods (Basel, Switzerland), 2022, 11, 762. DOI: https://doi.org/10.3390/foods11050762
Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzi ´nska, M. Appl. Sci. 2018, 8, 2606. DOI: https://doi.org/10.3390/app8122606
Santos-Silva, J.; Bessa, R.; Santos-Silva, F. Livest. Prod. Sci. 2002, 77, 187–194. DOI: https://doi.org/10.1016/S0301-6226(02)00059-3
Ghassemi-Golezani, K.; Farhangi-Abriz, S. Russ. J. Plant Physiol. 2018, 65, 229–236. DOI: https://doi.org/10.1134/S1021443718020115
Colombo, M. L.; Rise, P.; Giavarini, F.; De Angelis, L.; Galli, C.; Bolis, C. L. Plant Foods Hum. Nutr, 2006, 61, 64–69. DOI: https://doi.org/10.1007/s11130-006-0015-7

Downloads
Published
Issue
Section
License
Copyright (c) 2024 Emmanuel U. Ejiofor, Alwell C. Ako, Maxwell T. Kube, Ernest C. Agwamba, Chinweuba Alala, Kelvin Maduabuchi, Maureen Ejiofor

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








