Synthesis of Ferrocene Based Schiff Bases Possessing Different Metal Ion Sensing Aptitude and Partaking Antimicrobial Activity
DOI:
https://doi.org/10.29356/jmcs.v66i3.1677Keywords:
Unsymmentrical Schiff bases, ferrocene, cation sensors, azomethine, binding attitude, molecular dockingAbstract
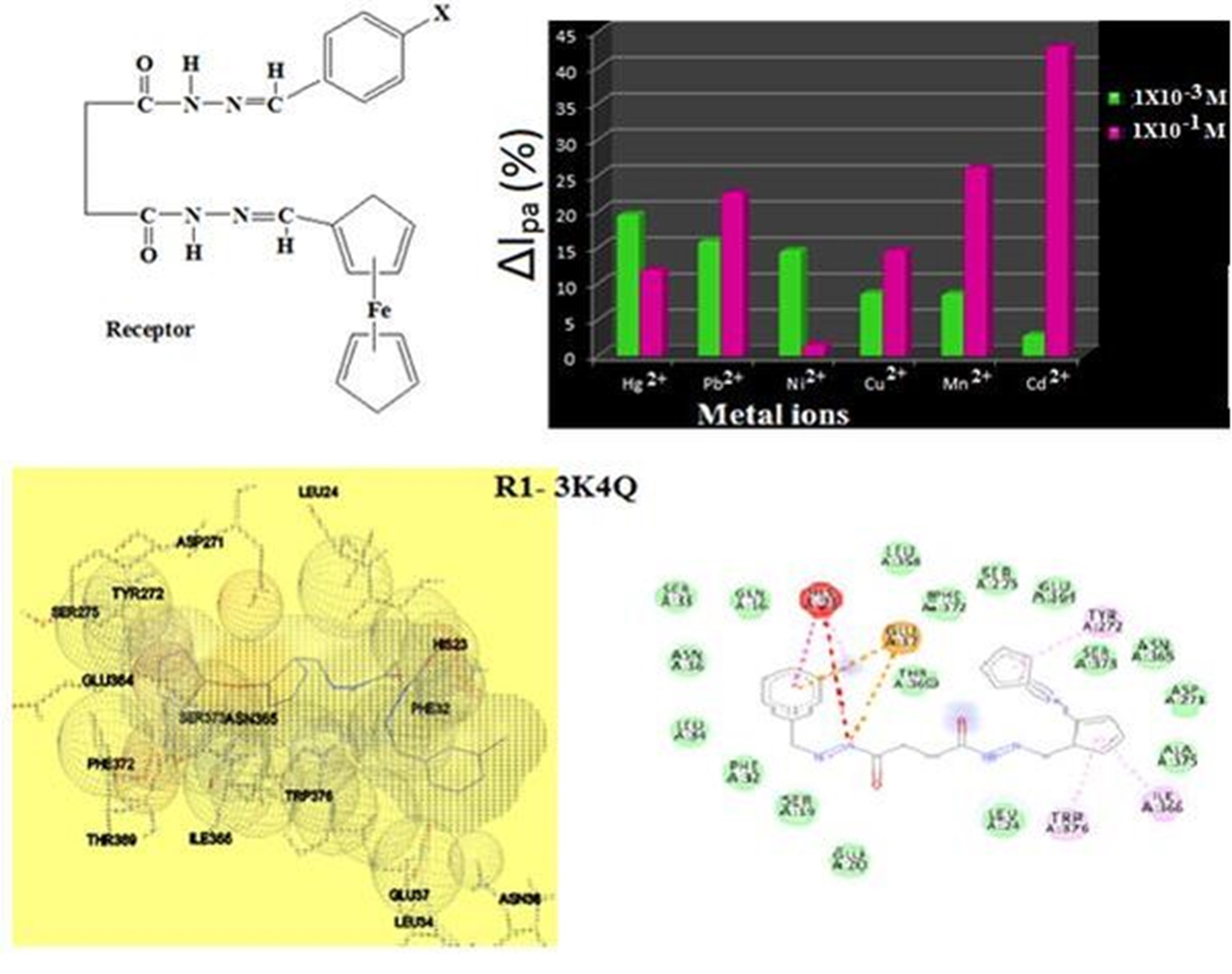
Abstract. Schiff bases comprised of highly reactive ferrocene derivatives and normal aromatic moiety have been prepared successfully. Spectral variations noticed in the spectra of newly synthesized receptors for the addition of different metal ions discloses the multi metal ion sensing ability of the prepared sensors. Harmonization of Cu2+ ions with receptor originate as MLCT band in the visible region. Shrewdness made from the data obtained from cyclic voltammetry studies give an idea about the concentration of metal ions needed for effective sensing. In vitro antimicrobial studies and H- bond energy calculation for the interaction between the above sensory materials and proteins of selected microorganisms using molecular docking studies disclosures the antifungal activity of newly prepared materials.
Resumen. Bases de Schiff derivadas de grupos ferrocenilos altamente reactivos y grupos aromáticas fueron preparadas exitosamente. La habilidad de los sistemas como sensores para detectar diversos iones metálicos se vió en la variación de las características observadas en sus espectors. La interacción de iones Cu2+ con el receptor produce una banda MLCT en la región visible. Los estudios de voltametría cíclica indican la concentración de los iones metálicos necesaria para una detección eficiente. Estudios antimicrobianos in vitro y cálculos de la energía de puentes de hidrógeno para las interacciones entre los sensores (bases de Schiff) y las proteínas de microorganismos selectos, basados en estudios de acoplamiento molecular, confirman la actividad antifúngica de los nuevos compuestos reportados.
Downloads
References
Osório, M. V.;, Marques, S. S.; Oliveira, H. M.; Barreiros, L.; Segundo, M. A. J. Food Compos. Anal. 2016, 45, 141–146. DOI: https://doi.org/10.1016/j.jfca.2015.10.007.
Frank, C. B.; Scott, R. B.; Kiril D. H.; Russell, G. B.; Evan, Taylor, D. A.; Hoffman. Maced. J. Chem. Chem. Eng. 2020, 39, 119–127. DOI: https://doi.org/10.20450/mjcce.2020. DOI: https://doi.org/10.20450/mjcce.2020.2088
Chiu-Hsien, Wu.; Guo-Jhen, J.; Kai-Wei, C.; Zu-Yin ,D.; Yu-Ning, L.; Kuen-Lin, C.; Chien-Chung, J. Sensors. 2018, 18, 163-171. DOI: https://doi.org/10.3390/s18010163.
Mayer, M.; Baeumner, A. J. Chem. Rev. 2019, 119, 7996–8027. DOI; https://doi.org/10.1021/acs.chemrev.8b00719.
Johnson, D. A.; Curtis, M. R.; Wallace, J. K. Chemosensors. 2019, 7, 1–48. DOI: https://doi.org/10.3390/chemosensors7020022.
Vedamalai, M.; Kedaria, D.; Vasita, R.; Moric, S.; Gupta, I. Dalton Trans. 2016, 45, 2700–2708. DOI: https://doi.org/10.1039/C5DT04042F.
Qi, X.; Jun, E. J.; Xu, L. J. Org. Chem. 2006, 71, 2881-2884. DOI: https://dx.doi.org/10.1021/jo052542a. DOI: https://doi.org/10.1021/jo052542a
Ingle, A. P.; Paralikar, P.; Shende, S.; Gupta, I.; Biswas, J. K.; da Silva Martins, L. H.; Rai, M. Biomedical Applications of Metals. 2018, 95–112. DOI: https://doi.org/10.1007/978-3-319-74814-6_4
Desguin, B.; Fellner, M.; Riant, O.; Hu, J.; Hausinger, R.P.; Hols, P.; Soumillion, P. J. Biol. Chem. 2018, 293, 12303–12317. DOI: https://doi.org/10.1074/jbc.RA118.003741.
Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M. S.; Catalano, A; Int. J. Environ. Res.Public Health. 2020, 17, 679.DOI: https://doi.org /10.3390/ijerph17030679. DOI: https://doi.org/10.3390/ijerph17030679
Zambelli, B.; Uversky, V.N.; Ciurli, S. BBA Proteins Proteom. 2016, 1864, 1714–1731. DOI: https://doi.org/10.1016/j.bbapap.2016.09.008
Mehrdad, R. R.; Mehravar R. R.; Sohrab, K.; Ali-akbar, M. Caspian J. Intern Med .2017, 8,135-145. DOI: https://doi.org/10.22088/cjim.8.3.135
Zhu, W.; Richards, N. G. J. Essays Biochem. 2017, 61, 259–270. DOI: https://doi.org/10.1042/ebc20160070
Freeland-Graves, J. H.; Mousa, T. Y.; Kim, S. J. Trace Elem. Med. Biol., 2016, 38, 24–32. DOI: https://doi.org/10.1016/j.jtemb.2016.05.004.
Hariharan, G.; Purvaja, R.; Ramesh, R. Environ. Toxicol. 2016, 31, 24–43. DOI: https://doi.org/10.1002/tox.22019.
Wani, A. L.; Ara, A.; Usmani, J. Interdiscip. Toxicol. 2015, 8, 55–64. DOI: https://doi.org/10.1515/intox-2015-0009.
Gidlow, D. A. Occup. Med. 2015, 65, 348–356. DOI: https://doi.org/10.1093/occmed/kqv018.
Dubar, F.; Egan, T. J.; Pradines, B.; Kuter, D.; Ncokazi, K. K.; Forge, D.; Biot, C. ACS Chemical Biology, 2011, 6, 275–287.DOI: https://doi.org/10.1021/cb100322v.
Gupta, S. R.; Mourya, P.; Singh, M. M.; Singh, V. P. J. Organomet. Chem. 2014, 767, 136–143. DOI: https://doi.org/10.1016/j.jorganchem.2014.05. DOI: https://doi.org/10.1016/j.jorganchem.2014.05.038
Hranjec, M.; Starčević, K.; Pavelić, S. K.; Lučin, P.; Pavelić, K.; Karminski Zamola, G. Eur. J. Med. Chem. 2011, 46, 2274–2279. DOI: https://doi.org/10.1016/j.ejmech.2011.03.008.
Mohamed, G. G.; Mahmoud, W. H.; Diab, M. A.; El-Sonbati, A. Z.; Abbas, S. Y. J. Mol. Struct. 2019, 1-15. DOI: https://doi.org/10.1016/j.molstruc.2019.01.00.
Farouk, K., Mohamad, K. C.; Wail Al. Z. ISRN Org. Chem. 2012, 8, 208284. DOI: https://doi.org/10.5402/2012/208284.
Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Food Microbiol.. 2004, 21, 33-42. DOI: https://dx.doi.org/10.1016/s0740-0020(03)00046-7. DOI: https://doi.org/10.1016/S0740-0020(03)00046-7
Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M. F.; Belew, R. K.; Goodsell, D.S.; Olson, A.J. J. Comput. Chem. 2009, 30, 2785- 2791. DOI: https://dx.doi.org/10.1002/jcc.21256. DOI: https://doi.org/10.1002/jcc.21256
Gryaznova, T. P.; Katsyuba, S. A.; Milyukov, V.A.; Sinyashin, O.G. J. Organomet. Chem. 2010, 695, 2586- 2595. DOI: https://dx.doi.org /10.1016/j.jorganchem.2010.08.031. DOI: https://doi.org/10.1016/j.jorganchem.2010.08.031
Mandewale, M. C.; Bapu,T.; Nivid , Y.; Ram Jadhav A. N.; Yamgar, R. J. Saudi Chem. Soc. 2016, 22, 218-228. DOI: https://dx.doi.org/10.1016/j.jscs.2016.04.003. DOI: https://doi.org/10.1016/j.jscs.2016.04.003
Berna,C Periodcals Eng. Nat. Sci. 2017, 5, 237-244. DOI: https://dx.doi.org/10.21533/pen.v5i2.139 DOI: https://doi.org/10.21533/pen.v5i2.139
Benramdane, R.; Benghanem, F.; Aliourari.; Keraghel, S.; Bouet, G. J. Coord. Chem, 2015, 68, 560-572. DOI: https://dx.doi.org/10.1080/00958972.2014.994514. DOI: https://doi.org/10.1080/00958972.2014.994514
Jinghui, C.; Xiaofeng, M.; Yuhui, Z.; Jiaoyan, L.; Xiangge, Z.; Haifeng, X. Inorg. Chem. 2014, 53, 3210−3219. DOI: https://doi.org/10.1021/ic5000815
Rampal, P.; Rakesh Kumar, G.; Mohammad, S.; Biswajit, M.; Arvind, M.; Daya Shankar, P. Inorg. Chem. 2012, 51, 298−311. DOI: https://doi.org/10.1021/ic201663m.
Schrage, B. R.; Zhao, Z.; Boika, A.; Ziegler, C. J. J. Org. Chem. 2019, 897, 23–31.DOI: https://doi.org/10.1016/j.jorganchem.2019.06.023.
Kamatchi, P.; Selvaraj, S.; Kandaswamy, M. Polyhendron. 2005, 24, 900-908. DOI: https://doi.org/10.1016/j.poly.2005.02.012.
Kamal, A S.; Kuma.r, S.; Kumar, V.; Mahajan, R.K. Sens. Actuators B. 2015, 22, 370-378. DOI: https://dx.doi.org/10.1016/j.snb.2015.06.147. DOI: https://doi.org/10.1016/j.snb.2015.06.147
John, M. W. S.; Jura, W. H. Can. J. Chem. 1967, 45, 2375-2384. DOI: https://doi.org/10.1139/v67-385.
Rebecca, Y. L.; Allen, J. B. J. Phys. Chem. B. 2003, 107, 5036-5042. DOI: https://doi.org/10.1021/jp034578h.
Hassan, A.S.; Askar, A.A.; Nossier, E.S.; Naglah, A.M.; Moustafa, G.O.; Al-Omar, M.A. Molecules. 2019, 24, 3130. DOI: https://doi.org/10.3390/molecules24173130.
Pozzi, C.; Ferrari, S.; Cortesi, D.; Luciani, R.; Stroud, R.M.; Catalano, A.; Costi, M.P.; Mangani, S. Acta Cryst. D 2012, 68, 1232–1241. DOI: https://doi.org/10.1107/S0907444912026236.
Magalhães, T. F. F.; da Silva, C. M.; Dos Santos, L. B. F.; Santos, D. A.; Silva, L. M.; Fuchs, B. B.; Mylonakis, E.; Martins, C. V. B. Lett. Appl. Microbiol. 2020, 71, 490–497. DOI: https://doi.org/10.1111/lam.13356.
Hamad, A.; Chen, Y.; Khan, M. A.; Jamshidi, S.; Saeed, N.; Clifford, M.; Hind, C.; Sutton, J. M.; Rahman, K.M. Microbiol. Open. 2021, 10, e1218. DOI: https://doi.org/10.1002/mbo3.1218.

Downloads
Published
Issue
Section
License
Copyright (c) 2022 Kamatchi Selvaraj P, Selvaraj Shunmugaperumal, Saranya Dhasarathan

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









