Synthesis, Structural Analysis and Antimicrobial Screening of Mn(II) Complexes of Schiff Bases
DOI:
https://doi.org/10.29356/jmcs.v66i1.1621Keywords:
Schiff bases, Mn (II) complex, antibacterial, antifungalAbstract
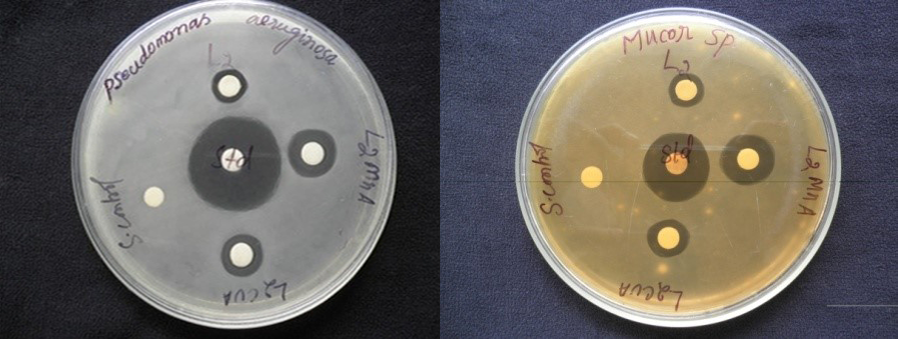
Abstract. Mn(II) complexes of Schiff bases 4-((3-ethoxy-2-hydroxybenzylidene)amino)-N-(pyridin-2-yl)benzenesulfonamide (HL2) and 4-((3-ethoxy-2-hydroxybenzylidene)amino)-N-(pyrimidin-2- yl)benzenesulfonamide (HL3) were synthesized. The Schiff bases HL2 and HL3 and their complexes were characterized by analytical, conductance, magnetic susceptibility measurements, infrared, ultraviolet-visible, thermal analysis, and EI mass techniques. The spectral data of the complexes have revealed the bidentate complexing nature of the Schiff base ligand through phenoxide ion and azomethine nitrogen atoms. The antibacterial activities of complexes were tested against gram-positive bacterial species Pseudomonas aeruginosa (NCIM 2036) and fungal species Aspergillus niger (NCIM 105) and Mucor sp. (NCIM 108) by disc diffusion method.
Keywords: Schiff bases; Mn(II) complex; antibacterial; antifungal.
Resumen. Los complejos de Mn(II) de las bases de Schiff 4-((3-etoxi-2-hidroxibencilideno)amino)-N-(piridin-2-il)bencenosulfonamida (HL2) y 4-((3-etoxi-2-hidroxibencilideno)amino)-N-(pirimidin-2- il)bencenosulfonamida (HL3) fueron sintetizados. Las bases de Schiff HL2 and HL3 y sus complejos fueron caracterizados por métodos analíticos, conductancia, susceptibilidad magnética, espectroscopia infrarroja y UV-vis, termogravimetría, y espectrometría de masas por impacto enectrónico. Los datos espectroscópicos obtenidos para los complejos corroboraron la coordinación bidentada de los ligantes de base de Schiff a través del ion fenóxido y el átomo de nitrógeno del grupo azometino. La actividad antibacterial de los complejos se evaluó contra cepas bacterianas gram-positivas Pseudomonas aeruginosa (NCIM 2036) y contra especies fúngicas Aspergillus niger (NCIM 105) y Mucor sp. (NCIM 108) utilizando el método de difusión en disco.
Downloads
References
C. Hansch, G.; Sammes, J. B.; Taylor, Comprehensive Medicinal Chemistry, Pergamon Press: Oxford 2. Chapter 7.1, 1990.
Vree, T. H. Clinical Pharmacokinetics of sulfonamides and their metabolities. Karger Basel 1987, 37.
Spinu, C.; Kriza, A. Acta Chim. 2000, 47, 179-185. DOI: https://doi.org/10.1023/A:1014014631732
Qu, Y.; Wang, C.; Wu, Y. C.; Zhao, K.; Wu, H. L. J. Appl. Spectrosc. 2000, 87, 429-436. DOI: https://doi.org/10.1007/s10812-020-01018-x
Ramesh, M.; Chandrasekhar, K.B.; Reddy Hussain, K. Indian J. Chem A. 2000, 39, 1337-1339.
Kaczmarek, M. T.; Jastrz?b, R.; Ho?derna-K?dzia, E.; Radecka-Paryzek, W. Inorg. Chim. Acta. 2009, 362, 3127-3133. DOI: https://doi.org/10.1016/j.ica.2009.02.012
Gomathi, V.; Selvameena, R.; Subbalakshmi, R.; Valarmathy, G. OJC. 2013, 29, 533-538. DOI: https://doi.org/10.13005/ojc/290220
Gull, P.; Hashmi, A. A. J. Braz. Chem. Soc. 2015, 26, 1331-1337.
Valarmathy, G.; Subbalakshmi, R.; Selvameena, R.; Gomathi, V. OJC. 2012, 29, 315-320.
Rama, I.; Usha, V. Asian J. Chem. 2013, 25, 3225-3228. DOI: https://doi.org/10.14233/ajchem.2013.13596
Ramakrishna Reddy, K.; Madhusudan, K. N.; Reddy, R.; Mahindra, K. Indian J. Chem Sec A. 2006, 45, 377-380.
Gomathi, V.; Selvameena, R. Main Group Chem. 2013, 12, 275-284. DOI: https://doi.org/10.3233/MGC-130107
Gomathi, V.; Selvameena, R. Int. J. Sci. Res. 2013, 2, 24-25. DOI: https://doi.org/10.15373/22778179/MAR2013/9
Gomathi, V.; Selvameena, R. Asian J. Chem. 2013, 25, 2083-2086 DOI: https://doi.org/10.14233/ajchem.2013.13323
Selma, Y. Russ J Gen Chem. 2014, 84, 1819-1222. DOI: https://doi.org/10.1134/S1070363214090308
Ferro, J. R. Low frequency vibrations of inorganic and coordination compound, New York, John Wiley, 1971. DOI: https://doi.org/10.1007/978-1-4684-1809-5
Maurya, R. C.; Chourasia, J.; Sharma, P. Indian J. Chem. 2007, 46, 1594-1604,
Maurya, R. C.; Pandey, A.; Sutradhar, D. Indian J. Chem. 2004, 46, 763-769.
Kavitha, S.; Singh, D. P. Vikas, K. Indian J. Chem Techn. 2017, 24, 534-537.
Halli, M. B.; Vijalaxmi, B. P. Indian J. Chem A. 2011, 50, 664- 668.
Safyah Bakare, B. Polish J. Chem. 2019, 21, 26-34. DOI: https://doi.org/10.2478/pjct-2019-0026
Bodhaei, M. D.; Mohebi, S. J. Chem. Res. 2001, 224-228.
Matin, S. J.; Khojasteh, R. R. Russ J. Gen. Chem. 2015, 85, 1763- 1768. DOI: https://doi.org/10.1134/S1070363215070312
Razieh A.; Mohammad Azarkishv.; Tahereh, S. J. Mex. Chem. Soc. 2014, 58, 173-179.
Vidyavati, R.; Nirdosh, P.; Angadi, S. D.; E- J. Chem. 2008, 5, 577-583. DOI: https://doi.org/10.1155/2008/170631
Maurya, R. C.; Chourasia, J. ; Rajak, D.; Malik, B. A.; Mir, J. M.; Jain, N.; Batalia, S. Arabian J. Chem. 2016, 9, S1084-1089. DOI: https://doi.org/10.1016/j.arabjc.2011.12.012
Lu, K. H.; Kia, Q. H.; Zhan, H. J.; Yuan, H. X.; Ye, C. P.; Su, K. X.; Xu, G.; J. Mol. Catal. A: Chem. 2006, 250, 62–69 DOI: https://doi.org/10.1016/j.molcata.2006.01.055
Karthikeyan, G.; Mohanraj, K.; Elango, K.P.; Girishkumar, K. Russ. J. Coord. Chem. 2006, 32, 380-385. DOI: https://doi.org/10.1134/S1070328406050113
Selvam, P.; Chandramohan, M.; Clercq, E.D.; Myriam, W.; Christophe, P. Eur. J. Pharm. Sci. 2001, 14, 313-318. DOI: https://doi.org/10.1016/S0928-0987(01)00197-X
Raman, N.; Raja. S. J.; Sakthivel, A. J. Coord. Chem. 2009, 62, 691-709. DOI: https://doi.org/10.1080/00958970802326179
Lever, A.B.P., “In Inorganic Electronic Spectroscopy”, 2nd ed, Elsevier, Amsterdam, 1984, 449.
Philip, V.; Suni, V.; Prathapchandra Kurup, M. R. P.; Nethaji, M. Spectrochim. Acta A. 2006, 64, 171-178. DOI: https://doi.org/10.1016/j.saa.2005.07.013
Batra, G.; Mathur, P. Transit. Metal Chem. 1994, 19, 160-165. DOI: https://doi.org/10.1007/BF00161880
Sulekh, C.; Savitha, B.; Rita, N.; Kushal, Q.; Saroj, K.; Sharma, K.; Spectrochim. Acta A., 2013, 113, 16-23.
Li, F.; Feterl, M.; Mulayana, Y.; Warner, J. M.; Collins, J. G.;Keene, F. R. J. Antimicrob. Chemother. 2012, 67, 2686-2695. DOI: https://doi.org/10.1093/jac/dks291
Sharaby, C. M. Spectrochim. Acta A, 2007, 66, 1271–1278. DOI: https://doi.org/10.1016/j.saa.2006.05.030
Naeimi, H.; J. Antimicrob. Chemother Moradian, M. J. Coord Chem. 2010, 63, 450-456.
Revanasiddappa, M.; Suresh, T.; Khasim, S.; Raghavendray, S. C.; Basavaraja, C.; Angadi, S. D. E-J. Chem. 2008, 5, 395–403. DOI: https://doi.org/10.1155/2008/328961
Anjaneyulu, Y.; Rao, P. R. Synth. React. Inorg. Met.-Org. Chem. 1986, 16, 257-261.
Dharmaraj, N.; Viswanathamurthi, P. Natarajan, K. Transit. Matal Chem. 2007, 26, 105. DOI: https://doi.org/10.1023/A:1007132408648
Mahajan, K.; Fahmi, N.; Singh, R. V. Indian J. Chem. 2007, 46, 1221–1225.
El-Sherif, A. A.; Eldebss, T. M. A.; Spectrochim. Acta A. 2011, 79, 1803–1814. DOI: https://doi.org/10.1016/j.saa.2011.05.062
Bagihalli, G. B.; Avaji, P. G.; Patil, S. A.; Badami, P. S. Eur. J. Med. Chem. 2008, 43, 2639–2649. DOI: https://doi.org/10.1016/j.ejmech.2008.02.013

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








