A Novel Efficient MPEG-Chitosan/HA Biopolymer for Adsorption of the Anticancer SN-38 Liquid Dispersions: Kinetics, Thermodynamic and Ex-Vivo Release Evaluation
DOI:
https://doi.org/10.29356/jmcs.v65i4.1505Keywords:
Nanoparticles, adsorption, kinetics, thermodynamics, ex-vivo releaseAbstract
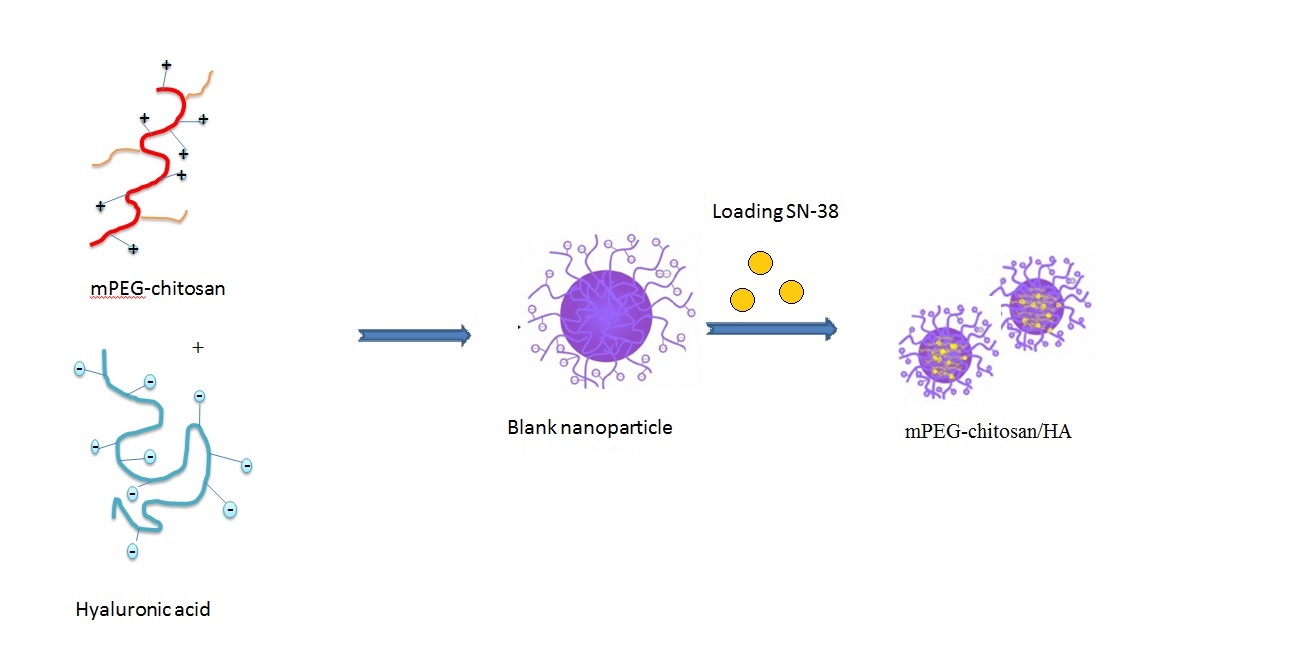
Abstract. Amongst several drug delivery schemes for perfect drug delivery comprise biocompatibility, selective aiming of cancer cells, low-cost, and safe process of nanoparticle preparation. In this work, a new mPEG-chitosan/HA biopolymer was prepared as adsorbent nanoparticles (mNPs) for an efficient drug delivery system. The mNPs was synthesized by conjugating poly (ethylene glycol) methyl ether (mPEG) to chitosan and prepared through ionic gelation between mPEG-chitosan and hyaluronic acid (HA). The prepared mNPs were used to adsorption/release of 7-ethyl-10-hydroxycamtothecin (SN-38) from its liquid dispersions. The mNPs adsorbent was characterized by Fourier transforms infrared spectroscopy (FT-IR), differential scanning calorimetry (DSC), scanning electron microscopy (SEM). The results demonstrated that the adsorption isotherm of SN-38 on mNPs follows Langmuir model, and the adsorption capacity was 346.511 mg g-1. Besides, the pseudo-first order kinetic well fitted the equilibrium data. Further, thermodynamic parameters including ΔH, ΔG and ΔS were calculated which demonstrated that the physical spontaneous adsorption was prevailing. In addition, the ex- vivo release of SN-38 from mNPs were in good agreement with Korsmeyer-Peppas equation indicating the drug release process was governed by diffusion phenomena. The above results revealed that mNPs containing SN-38 was a good candidate for the drug delivery systems.
Resumen. Dentro de las diferentes propiedades importantes de los sistemas de liberación de fármacos se encuentran la biocompatibilidad, el ataque selectivo a las células cancerosas, el bajo costo y los procesos adecuados de preparación de nanopartículas. En este trabajo, un nuevo biopolímero de mPEG-chitosan/HA se preparó en la forma de nanopartículas (mNPs) para el uso como un sistema de liberación controlada de fármacos. Las nanopartículas se sintetizaron incorporando el éter metílico de poli(etilenglidol) al quitosano, y se prepararon a través de la gelación iónica entre el mPEG-quitosano y el ácido hialurónico (HA). Las nanopartículas así preparadas se probaron en su efectividad para la absorción y liberación de 7-etil-10-hidroxicamtotecina (SN-38) en forma de dispersiones líquidas. El absorbente hecho a partir de las nanopartículas se caracterizó mediante espectroscopía infrarroja de transformada de Fourier (FT-IR), calorimetría diferencial de barrido (DSC) y microscopía electrónica de barrido (SEM). Se encontró que la isoterma de adsorción de la muestra de nanopartículas conteniendo SN-38 se ajusta al modelo de Langmuir, siendo el valor de la capacidad de adsorción de 346.511 mg g-1. El modelo cinético de seudo primer orden se ajusta adecuadamente a los datos obtenidos al equilibrio. Más aún, los parámetros termodinámicos tales como ΔH, ΔG and ΔS se pudieron calcular, lo que indica que la adsorción física espontánea es el mecanismo que prevalece. Además, los datos de liberación ex- vivo de SN-38 a partir de las nanopartículas se pueden ajustar a la ecuación de Korsmeyer-Peppas, indicando que el proceso de liberación del fármaco está gobernado por un proceso de difusión. Los resultados anteriores indican que el sistema de nanopartículas conteniendo SN-38 es un buen candidato para desarrollar un sistema de liberación controlada de fármacos.
Downloads
References
Fang, J.-Y.; Hung, C.-F.; Hua, S.-C.; Hwang, T.-L. Ultrasonics. 2009, 49, 39-46 DOI: https://doi.org/10.1016/j.ultras.2008.04.009
Ebrahimnejad, P.; Dinarvand, R.; Sajadi, A., J. Food Drug Anal. 2009, 17.
Einafshar, E.; Asl, A. H.; Nia, A. H.; Mohammadi, M.; Malekzadeh, A.; Ramezani, M. Carbohydr. Polym. 2018, 194, 103-110. DOI: https://doi.org/10.1016/j.carbpol.2018.03.102
Sahoo, N. G.; Bao, H.; Pan, Y.; Pal, M.; Kakran, M.; Cheng, H. K. F.; Li, L.; Tan, L. P. Chem. Commun. 2011, 47, 5235-5237. DOI: https://doi.org/10.1039/c1cc00075f
Mohammady, H.; Dinarvand, R.; Esfandyari Manesh, M.; Ebrahimnejad, P. Nanomed. J. 2016, 3, 159-168.
Yang, Z.; Luo, H.; Cao, Z.; Chen, Y.; Gao, J.; Li, Y.; Jiang, Q.; Xu, R.; Liu, J., Nanoscale. 2016, 8, 11543-11558. DOI: https://doi.org/10.1039/C6NR01749E
England, R. M.; Hare, J. I.; Barnes, J.; Wilson, J.; Smith, A.; Strittmatter, N.; Kemmitt, P. D.; Waring, M. J.; Barry, S. T.; Alexander, C. J. Controlled Release. 2017, 247, 73-85. DOI: https://doi.org/10.1016/j.jconrel.2016.12.034
Lee, S.-Y.; Yang, C.-Y.; Peng, C.-L.; Wei, M.-F.; Chen, K.-C.; Yao, C.-J.; Shieh, M.-J. Biomaterials. 2016, 86, 92-105. DOI: https://doi.org/10.1016/j.biomaterials.2016.01.068
Butler, K. S.; Durfee, P. N.; Theron, C.; Ashley, C. E.; Carnes, E. C.; Brinker, C. J. Small. 2016, 12, 2173-2185. DOI: https://doi.org/10.1002/smll.201502119
Sharifi, F.; Nazir, I.; Asim, M. H.; Jahangiri, M.; Ebrahimnejad, P.; Matuszczak, B.; Bernkop-Schnürch, A. J. Mol. Liq. 2019, 291, 111285. DOI: https://doi.org/10.1016/j.molliq.2019.111285
Toole, B. P. Nat. Rev. Cancer. 2004, 4, 528-539. DOI: https://doi.org/10.1038/nrc1391
Sadeghi Ghadi, Z.; Ebrahimnejad, P. J. Microencapsulation. 2019, 36, 169-179. DOI: https://doi.org/10.1080/02652048.2019.1617360
Dang, J. M.; Leong, K. W. Adv. Drug Delivery Rev. 2006, 58, 487-499. DOI: https://doi.org/10.1016/j.addr.2006.03.001
Al-Qadi, S.; Alatorre-Meda, M.; Zaghloul, E. M.; Taboada, P.; Remunán-López, C., Colloids Surf. 2013, 103, 615-623. DOI: https://doi.org/10.1016/j.colsurfb.2012.11.009
Mir, M.; Ebrahimnejad, P. Nanoscience & Nanotechnology-Asia. 2014, 4, 80-87. DOI: https://doi.org/10.2174/2210681205666150515000224
Dong, Y.; Feng, S.-S. Biomaterials. 2004, 25, 2843-2849. DOI: https://doi.org/10.1016/j.biomaterials.2003.09.055
Venkataraman, S.; Hedrick, J.; Ong, Z. Adv. Drug Delivery Rev. 2011, 63, 1228. DOI: https://doi.org/10.1016/j.addr.2011.06.016
O'neill, V.; Twelves, C. Br. J. Cancer. 2002, 87, 933-937. DOI: https://doi.org/10.1038/sj.bjc.6600591
Dramou, P.; Fizir, M.; Taleb, A.; Itatahine, A.; Dahiru, N. S.; Mehdi, Y. A.; Wei, L.; Zhang, J.; He, H. Carbohydr. Polym. 2018, 197, 117-127. DOI: https://doi.org/10.1016/j.carbpol.2018.05.071
Itatahine, A.; Mehdi, Y. A.; Fizir, M.; Qi, M.; Dramou, P.; He, H. New J. Chem. 2018, 42, 1326-1336. DOI: https://doi.org/10.1039/C7NJ04609J
Kar, K. K. Carbon nanotubes: synthesis, characterization and applications. Research Publishing Service: 2011.
Matsumura, Y. Adv. Drug Delivery Rev. 2008, 60, 899-914. DOI: https://doi.org/10.1016/j.addr.2007.11.010
Chen, K.-J.; Tang, L.; Garcia, M. A.; Wang, H.; Lu, H.; Lin, W.-Y.; Hou, S.; Yin, Q.; Shen, C. K.-F.; Cheng, J. Biomaterials. 2012, 33, 1162-1169. DOI: https://doi.org/10.1016/j.biomaterials.2011.10.044
Zhuang, J.; Kuo, C.-H.; Chou, L.-Y.; Liu, D.-Y.; Weerapana, E.; Tsung, C.-K. ACS nano. 2014, 8, 2812-2819. DOI: https://doi.org/10.1021/nn406590q
Chi, Y.; Wang, Z.; Wang, J.; Dong, W.; Xin, P.; Bi, J.; Jiang, T.; Chen, C.-P. Colloid Polym. Sci. 2020, 298, 51-58. DOI: https://doi.org/10.1007/s00396-019-04581-8
Su, X.; Wu, L.; Hu, M.; Dong, W.; Xu, M.; Zhang, P. Biomed. Pharmacother. 2017, 95, 670-678. DOI: https://doi.org/10.1016/j.biopha.2017.08.123
Kulkarni, A. R.; Hukkeri, V. I.; Sung, H. W.; Liang, H. F. Macromol. Biosci. 2005, 5, 925-928. DOI: https://doi.org/10.1002/mabi.200500048
Yang, L.; Gao, S.; Asghar, S.; Liu, G.; Song, J.; Wang, X.; Ping, Q.; Zhang, C.; Xiao, Y. Int. J. Biol. Macromol. 2015, 72, 1391-1401. DOI: https://doi.org/10.1016/j.ijbiomac.2014.10.039
Wu, S.; Zhao, X.; Li, Y.; Du, Q.; Sun, J.; Wang, Y.; Wang, X.; Xia, Y.; Wang, Z.; Xia, L. Materials. 2013, 6, 2026-2042. DOI: https://doi.org/10.3390/ma6052026
Saremi, S.; Atyabi, F.; Akhlaghi, S. P.; Ostad, S. N.; Dinarvand, R. Int. J. Nanomed. 2011, 6, 119. DOI: https://doi.org/10.2147/IJN.S15500
Sun, X.; Zhu, D.; Cai, Y.; Shi, G.; Gao, M.; Zheng, M. Int. J. Nanomed. 2019, 14, 2115. DOI: https://doi.org/10.2147/IJN.S193783
Dada, A.; Olalekan, A.; Olatunya, A.; Dada, O. IOSR J. Appl. Chem. 2012, 3, 38-45.
Sharma, Y.; Srivastava, V.; Upadhyay, S.; Weng, C. Ind. Eng. Chem. Res. 2008, 47, 8095-8100. DOI: https://doi.org/10.1021/ie800831v
Alimohammady, M.; Jahangiri, M.; Kiani, F.; Tahermansouri, H. J. Environ. Chem. Eng. 2017, 5, 3405-3417. DOI: https://doi.org/10.1016/j.jece.2017.06.045
Davodi, B.; Jahangiri, M.; Ghorbani, M. Part. Sci. Technol. 2019, 1-12.
Kumar, V. Arabian J. Chem. 2019, 12, 316-329. DOI: https://doi.org/10.1016/j.arabjc.2016.11.009
Alimohammady, M.; Jahangiri, M.; Kiani, F.; Tahermansouri, H. Res. Chem. Intermed. 2018, 44, 69-92. DOI: https://doi.org/10.1007/s11164-017-3091-4
Khan, T. A.; Chaudhry, S. A.; Ali, I. J. Mol. Liq. 2015, 202, 165-175. DOI: https://doi.org/10.1016/j.molliq.2014.12.021
Anbia, M.; Mohammadi Nejati, F.; Jahangiri, M.; Eskandari, A.; Garshasbi, V. J. Sci., Islamic Repub. Iran. 2015, 26, 213-222.
Hidaka, M.; Yamasaki, K.; Okumura, M.; Ogikubo, T.; Iwakiri, T.; Setoguchi, N.; Nishida, K.; Nagai, K.; Ikenoue, T.; Arimori, K. Cancer Chemother. Pharmacol. 2007, 59, 321-328. DOI: https://doi.org/10.1007/s00280-006-0273-y
Guo, M.; Rong, W.-T.; Hou, J.; Wang, D.-F.; Lu, Y.; Wang, Y.; Yu, S.-Q.; Xu, Q. Nanotechnology. 2013, 24, 245101-245120. DOI: https://doi.org/10.1088/0957-4484/24/24/245101
Prasad, S.; Dangi, J. Artif. Cells, Nanomed., Biotechnol. 2016, 44, 1824-1834. DOI: https://doi.org/10.3109/21691401.2015.1105239
Malek, S. K.; Gabris, M. A.; Jume, B. H.; Baradaran, R.; Aziz, M.; Karim, K. J. B. A.; Nodeh, H. R. Daru, J. Pharm. Sci. 2019, 27, 9-20. DOI: https://doi.org/10.1007/s40199-018-0232-2
Akçay, G.; K?l?nç, E.; Akçay, M. Colloids Surf. A. 2009, 335, 189-193. DOI: https://doi.org/10.1016/j.colsurfa.2008.11.009
Morton III, S. A.; Keffer, D. J.; Counce, R.; DePaoli, D.; Hu, M.-C. J. Colloid Interface Sci. 2004, 270, 229-241. DOI: https://doi.org/10.1016/j.jcis.2003.08.006
Gereli, G.; Seki, Y.; Ku?o?lu, ?. M.; Yurdakoç, K. J. Colloid Interface Sci. 2006, 299, 155-162. DOI: https://doi.org/10.1016/j.jcis.2006.02.012
Karaca, S.; Gürses, A.; Ejder, M.; Aç?ky?ld?z, M. J. Colloid Interface Sci. 2004, 277, 257-263. DOI: https://doi.org/10.1016/j.jcis.2004.04.042
Choi, K. Y.; Min, K. H.; Yoon, H. Y.; Kim, K.; Park, J. H.; Kwon, I. C.; Choi, K.; Jeong, S. Y. Biomaterials. 2011, 32, 1880-1889. DOI: https://doi.org/10.1016/j.biomaterials.2010.11.010
Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S. W. A.; de Matas, M. Drug Delivery Transl. Res. 2019, 9, 721-734. DOI: https://doi.org/10.1007/s13346-019-00631-4
Gouda, R.; Baishya, H.; Qing, Z. J. Dev. Drugs. 2017, 6.
Walters, K. A.; Brain, K. R. Dermatological formulation and transdermal systems. In Dermatological and transdermal formulations, CRC Press, 2002, 337-418. DOI: https://doi.org/10.1201/9780824743239-10
Omidian, H.; Park, K., Introduction to hydrogels. In Biomedical applications of hydrogels handbook, Springer, 2010, 1-16. DOI: https://doi.org/10.1007/978-1-4419-5919-5_1
Korsmeyer, R. W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N. A. Int. J. Pharm. 1983, 15, 25-35. DOI: https://doi.org/10.1016/0378-5173(83)90064-9
Klech, C. M.; Simonelli, A. P. J. Membr. Sci. 1989, 43, 87-101. DOI: https://doi.org/10.1016/S0376-7388(00)82355-8
Peppas, N. A.; Narasimhan, B. J. Controlled Release. 2014, 190, 75-81. DOI: https://doi.org/10.1016/j.jconrel.2014.06.041
Kosmidis, K.; Argyrakis, P.; Macheras, P. J. Chem. Phys. 2003, 119, 6373-6377. DOI: https://doi.org/10.1063/1.1603731
Yamaura, M.; Camilo, R.; Sampaio, L.; Macedo, M.; Nakamura, M.; Toma, H. J. Magn. Magn. Mater. 2004, 279, 210-217. DOI: https://doi.org/10.1016/j.jmmm.2004.01.094
Agüeros, M.; Zabaleta, V.; Espuelas, S.; Campanero, M.; Irache, J. J. Controlled Release. 2010, 145, 2-8. DOI: https://doi.org/10.1016/j.jconrel.2010.03.012
Yue, X.; Qiao, Y.; Qiao, N.; Guo, S.; Xing, J.; Deng, L.; Xu, J.; Dong, A. Biomacromolecules. 2010, 11, 2306-2312. DOI: https://doi.org/10.1021/bm100410m
Ling, L.; Ismail, M.; Du, Y.; Xia, Q.; He, W.; Yao, C.; Li, X. Mol. Pharmaceutics. 2018, 15, 5479-5492. DOI: https://doi.org/10.1021/acs.molpharmaceut.8b00585
Zhang, Z.; Xu, Y.; Zhao, K.; Zhang, Y.; Chen, W.; Li, X.; Meng, Y.; Yang, D.; Wang, P.; Zhu, J. Med. Res. 2020, 3. DOI: https://doi.org/10.21127/yaoyimr20190008
Gan, M.; Zhang, W.; Wei, S.; Dang, H. Artif. Cells, Nanomed., Biotechnol. 2017, 45, 389-397. DOI: https://doi.org/10.3109/21691401.2016.1167700
Dinarvand, M.; Kiani, M.; Mirzazadeh, F.; Esmaeili, A.; Mirzaie, Z.; Soleimani, M.; Dinarvand, R.; Atyabi, F. Int. J. Biol. Macromol. 2015, 78, 112-121. DOI: https://doi.org/10.1016/j.ijbiomac.2015.03.066
Patil, A.; Nimbalkar, M.; Patil, P.; Chougale, A.; Patil, P. Mater. Today: Proc. 2020, 23, 437-443. DOI: https://doi.org/10.1016/j.matpr.2020.02.064
Chen, D.; Bi, J.; Wu, J.; Kumar, A. J. Inorg. Organomet. Polym. Mater. 2020, 30, 573-579. DOI: https://doi.org/10.1007/s10904-019-01188-y
Karki, N.; Tiwari, H.; Pal, M.; Chaurasia, A.; Bal, R.; Joshi, P.; Sahoo, N. G. Colloids Surf. 2018, 169, 265-272. DOI: https://doi.org/10.1016/j.colsurfb.2018.05.022

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









