Antimicrobial and Antioxidant Role of the Aerial Parts of Aconitum violaceum
DOI:
https://doi.org/10.29356/jmcs.v65i1.1310Keywords:
Aconitum violaceum, phytochemical, screening cytotoxic, antimicrobial; antioxidant, cholinesterase inhibitionAbstract
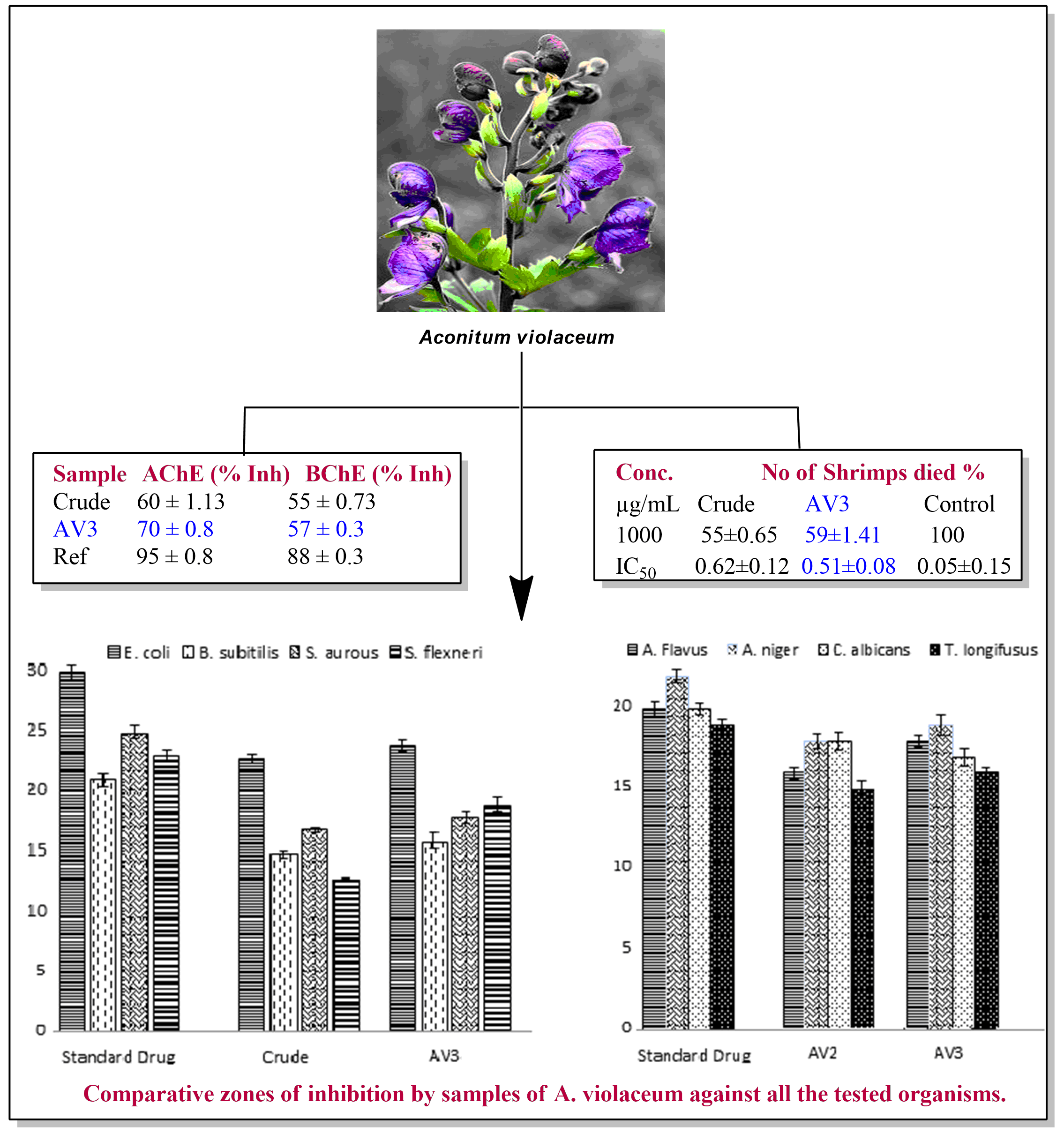
Abstract. In the current studies, crude and subsequent fractions of Aconitum violaceum aerial parts were screened for their toxicity, antimicrobial effects as well as antioxidant potential. Phytochemically, the plant is enriched in alkaloids alongside anthraquinones (present in ethyl acetate fraction (AV3) and saponins (detected in chloroform fraction (AV2). In Brine shrimp lethality assay, AV3 was the most potent (59 %) in killing Artemia naupili larvae at a dose of 1000 µg/mL. AV3 exhibited strongest antimicrobial effect against the bacteria E. coli (80 %) and S. flexneri (76 %) as well as against the tested fungi, A. niger (86 %) respectively. However, chloroform fraction (AV2) was the most effective (almost 90 %) antifungal against A. niger and C. albicans. Overall, strong antioxidant activity was observed for AV3 with IC50 values of 120.04 ± 0.4 µg/mL (65.4 ± 0.01 standard) in DPPH and 125.1 ± 0.3 µg/mL (2.0 ± 0.03 standard) in ABTS free radical assays. AV3 showed promising inhibition against acetylcholinesterase (AChE) 70 ± 0.8 % and butyrylcholinesterase (BChE) 57 ± 0.2 % at dose of 100 µg/mL thus confirming a potent invitro cholinesterase inhibitory effect. The overall results indicated strong biological potential of ethyl acetate fraction obtained form A. violaceum and a possible new therapeutic source could be formulated from its pure isolates.
Resumen.
En este estudio, se analizaron debido a su toxicidad, las fracciones crudas y posteriores de las partes aéreas de Aconitum violaceum, sus efectos antimicrobianos y potencial antioxidante. Fitoquímicamente, la planta está enriquecida en alcaloides junto con antraquinonas (presentes en la fracción de acetato de etilo (AV3) y saponinas (detectadas en la fracción de cloroformo (AV2). En el ensayo de letalidad del camarón en salmuera, AV3 fue el más potente (59%) para matar las larvas de Artemia naupili a una dosis de 1000 μg / ml. AV3 mostró el efecto antimicrobiano más fuerte contra las bacterias E. coli (80 %) y S. flexneri (76 %), así como contra los hongos probados, A. niger (86 %) respectivamente. Sin embargo, la fracción de cloroformo (AV2) fue el antifúngico más eficaz (casi el 90%) contra A. niger y C. albicans. En general, se observó una fuerte actividad antioxidante para AV3 con valores de CI50 de 120.04 ± 0.4 μg / ml (65.4 ± 0,01 estándar) en DPPH y 125.1 ± 0.3 μg / mL (2.0 ± 0.03 estándar) en ensayos de radicales libres ABTS. AV3 mostró una inhibición prometedora contra la acetilcolinesterasa (AChE) 70 ± 0.8 % y la butirilcolinesterasa (BChE) 57 ± 0.2 % a una dosis de 100 μg / mL confirmando así un potente efecto inhibidor de la colinesterasa in vitro. Todos los resultados indicaron un fuerte potencial biológico de la fracción de acetato de etilo obtenida de A. violaceum y se podría formular una posible nueva fuente terapéutica a partir de sus aislados puros.Downloads
References
Mukherjee, T. Protection of Traditional Knowledge. In Medicinal Plants: Biodiversity, Sustainable Utilization and Conservation, Springer, Singapore, 2020, 135-142 DOI: https://doi.org/10.1007/978-981-15-1636-8_8
Newman, D.J.; Cragg, G.M. J. Nat. Prod. 2020, 83, 770-803 DOI: https://doi.org/10.1021/acs.jnatprod.9b01285
?u?an, N. A. Curr. Trend. Nat. Sci. 2018, 7, 28-39
Braca, A.; Fico, G.; Morelli, I.; De Simone, F.; Tomè, F.; De Tommasi, N. J. Ethnopharmacol. 2003, 86: 63-7 DOI: https://doi.org/10.1016/S0378-8741(03)00043-6
Gao, L. M.; Yan, L. Y; He, H. Y.; Wei, X. M. J. Integr. Plant Biol. 2006, 48, 364–369 DOI: https://doi.org/10.1111/j.1744-7909.2006.00178.x
Kunwar, R. M.; Shrestha, K. P.; Bussmann, R. W. J. Ethnobiol. Ethnomed. 2010, 6, 35. DOI: https://doi.org/10.1186/1746-4269-6-35
Kapoor, B.; Kaur, G.; Gupta, M.; Gupta, R. Asian J. Pharm. Clin. Res, 2017, 10, 407. DOI: https://doi.org/10.22159/ajpcr.2017.v10i11.20170
Masood, A.; Wang, Z.; Farman, A.; Song, Z.; Cox, R.; Khan, S. U. J. Med. Plants Res. 2013, 7, 3066-3070
Meyer, B.N.; Ferringni, N. R.; Puam, J. E.; Lacobsen, L. B.; Nichols, D. E.; McLaughlin, J. L. Planta Medica, 1982, 45, 31 DOI: https://doi.org/10.1055/s-2007-971236
Rahman, S.; Imran, M.; Muhammad, N.; Hassan, N.; Chisthi, A.; Khan, A.; Sadozai, K.; Khan, S. J.Med. Plants Res. 2011, 5, 5167-5171.
Duraipandiyan, V.; Ignacimuthu, S. J. Ethnopharmacol. 2009, 123, 494-498. DOI: https://doi.org/10.1016/j.jep.2009.02.020
Obied, H. K.; Allen, M. S.; Bedgood, D. R.; Prenzler, P. D.; Robards, K.; Stockmann, R. J. Agri. Food Chem., 2005, 53, 823-837. DOI: https://doi.org/10.1021/jf048569x
Tan, C.; Xue, J.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. J. Agri. Food Chem. 2014, 62, 6726-6735 DOI: https://doi.org/10.1021/jf405622f
Rocha, J. B.; Emanuelli, T.; Pereira, M. E. Acta Neurobio. Exp. 1993, 53, 431-431.
Abrantes, A. F.; Rocha, T. D.; Lima, A. B.; Cavalcanti, M. T. Ciência Rural, 2017, 47. DOI: https://doi.org/10.1590/0103-8478cr20160486
Khuda, F.; Iqbal, Z.; Khan, A.; Nasir, F.; Muhammad, N.; Khan, J. A.; Khan, M. S. Pak. J. Pharm. Sci. 2012, 25, 51-58.
Akal, N.; Bereswill, S.; Heimesaat, M. European J. Microbiol. Immunol. 2017, 7, 92-98. DOI: https://doi.org/10.1556/1886.2017.00001
Cheesman, M. J.; Ilanko, A.; Blonk, B.; Cock, I. E. Pharmacol. Rev. 2017, 11, 57. DOI: https://doi.org/10.4103/phrev.phrev_21_17
Kumari, A.; Promwichit, P. Am. J. Food Technol. 2009, 4, 192-200 DOI: https://doi.org/10.3923/ajft.2009.192.200
Ishtiyak, P.; Hussain, S. A. Studies on Ethno-Medicine, 2017, 11, 318-331. DOI: https://doi.org/10.1080/09735070.2017.1335123
Kadir, T.; Gümrü, B.; Uygun-Can, B. Arch. Oral. Biol. 2007, 52, 691-696. DOI: https://doi.org/10.1016/j.archoralbio.2006.12.008
Khan, S.; Khan., H. U.; Muhammad, A. R.; Khan. F. U.; Rehman, U. K. Inter. J. Adv. Res 2013, 8, 28-33.
Khan, S.; Khan, H.; Ali, F.; Ali, N.; Khan U. F.; Khan, U. S. Nat. Prod. Res. 2016, 30, 1335-1338. DOI: https://doi.org/10.1080/14786419.2015.1055743
Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Food Chem. 2005, 90, 333-340 DOI: https://doi.org/10.1016/j.foodchem.2003.09.013
Wong, D.; Bellyou, M.; Beauchet, O.; Montero-Odasso, M.; Annweiler, C.; Bartha, R. J. Alzheimer's Assoc., 2017, 13, 269-270. DOI: https://doi.org/10.1016/j.jalz.2017.06.2316
Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. J. Alzheimer's Disease, 2018, 62, 1319-1335. DOI: https://doi.org/10.3233/JAD-170732
Ali-Shtayeh, M. S.; Jamous, M. R.; Al-Shafie, H. J.; Elgharabah, A. W.; Kherfan, A. F.; Qarariah, H. K.; Herzallah, M. H. J. Ethnobiol. Ethnomed, 2008, 4, 1. DOI: https://doi.org/10.1186/1746-4269-4-13
Mehta, S. H.; Sudarshi, D.; Srikrishnan, K. A.; Celentano, D. D.; Vasudevan, K. C.; Anand, S.; Solomon, S. S. Addiction. 2012, 107, 349-358. DOI: https://doi.org/10.1111/j.1360-0443.2011.03602.x
Gauthier, S.; Emre, M.; Farlow, R. M.; Bullock, R.; Grossberg, T. G.; Potkin, G. S. Curr. Med. Res. Opin. 2003, 19, 707-714. DOI: https://doi.org/10.1185/030079903125002450

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









