Nanostructured Lipid Carriers-Chitosan Carrier System for Loading of Dipsacus Asper Essential Oil: Preparation, Characterization, Antioxidant Study
DOI:
https://doi.org/10.29356/jmcs.v70i1.2165Keywords:
Nanostructured lipid carriers, volatile oil, Dipsacus asper, storage stabilityAbstract
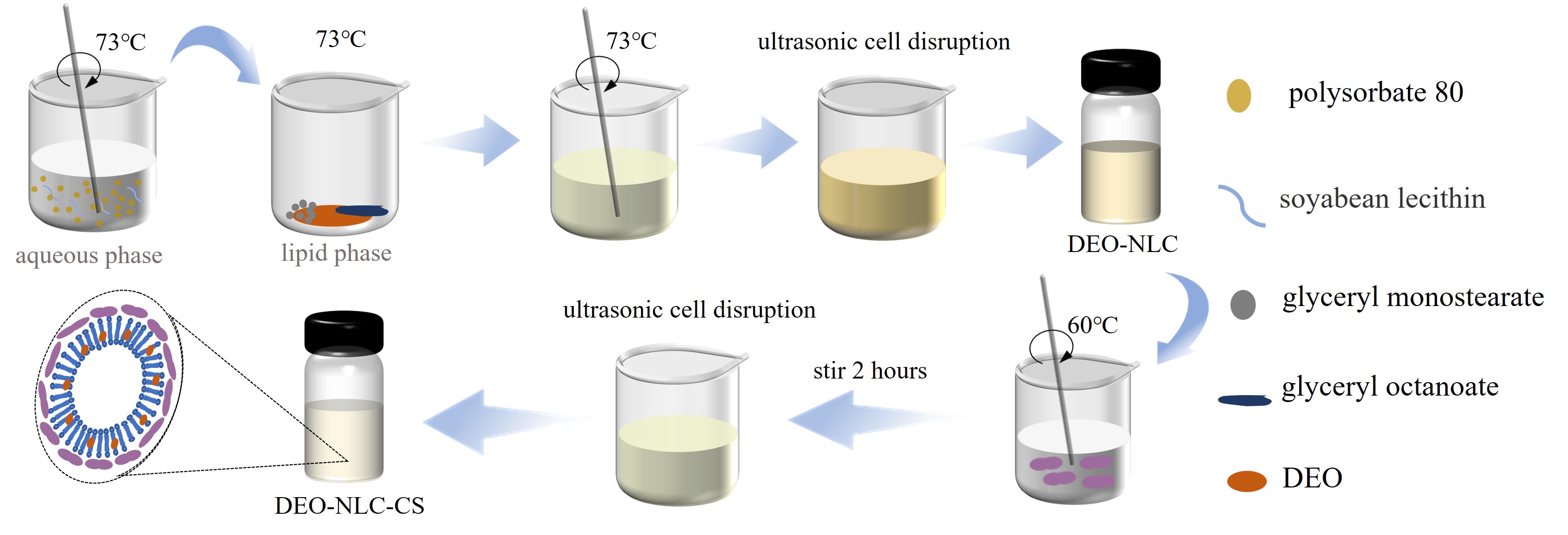
Abstract. Dipsacus asper essential oil (DEO) was encapsulated within a nanostructured lipid carrier (DEO-NLC), with chitosan (DEO-NLC-CS) subsequently applied as a surface coating. These carriers ' physicochemical and morphological properties, stability, in vitro release performance, and antioxidant activity were investigated. This study presents a new approach to address the challenges of Dipsacus essential oil's volatility and poor water solubility. The average diameter of DEO-NLC and DEO-NLC-CS were 68.90 ± 1.18 and 119.40 ± 1.40 nm, respectively, as determined by dynamic light scattering (DLS). Scanning electron microscope (SEM) and transmission electron microscope (TEM) confirmed both carriers were spherical-like coating structures, which confirmed the results of DLS. Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) showed the successful physical capture of DEO in DEO-NLC and DEO-NLC-CS. The X-ray diffractogram of DEO-NLC and DEO-NLC-CS exhibited a wide high-intensity peak at 2θ = 15~25°, indicating that DEO was entrapped within NLC. It has been confirmed through differential scanning calorimetry (DSC) that the chitosan matrix successfully encapsulated DEO. In vitro release studies showed that both exhibited good sustained release properties. The antioxidant studies showed that blank NLC, DEO-NLC, and DEO-NLC-CS have good 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH·) scavenging activities.
Resumen. El aceite esencial de Dipsacus asper (DEO) se encapsuló dentro de un portador lipídico nanoestructurado (DEO-NLC), y posteriormente se aplicó quitosano (DEO-NLC-CS) como recubrimiento de superficie. Se investigaron las propiedades fisicoquímicas y morfológicas, la estabilidad, el rendimiento de liberación in vitro y la actividad antioxidante de estos portadores. Este estudio presenta un nuevo enfoque para abordar los desafíos de la volatilidad y la mala solubilidad en agua del aceite esencial de Dipsacus. El diámetro promedio de DEO-NLC y DEO-NLC-CS fue 68,90 ± 1,18 y 119,40 ± 1,40 nm, respectivamente, según lo determinado por dispersión dinámica de luz (DLS). El microscopio electrónico de barrido (SEM) y el microscopio electrónico de transmisión (TEM) confirmaron que ambos portadores eran estructuras de recubrimiento esféricas, lo que confirmó los resultados de la DLS. La espectroscopia infrarroja de transformada de Fourier de reflectancia total atenuada (ATR-FTIR) mostró la captura física exitosa de DEO en DEO-NLC y DEO-NLC-CS. El difractograma de rayos X de DEO-NLC y DEO-NLC-CS mostró un pico amplio de alta intensidad en 2θ = 15~25°, lo que indica que el DEO quedó atrapado dentro de NLC. Se ha confirmado mediante calorimetría diferencial de barrido (DSC) que la matriz de quitosano encapsuló con éxito el DEO. Los estudios de liberación in vitro demostraron que ambos presentaban buenas propiedades de liberación sostenida. Los estudios de antioxidantes mostraron que los blancos NLC, DEO-NLC y DEO-NLC-CS tienen buenas actividades de eliminación del radical 1,1-difenil-2-picril-hidrazilo (DPPH·).
Downloads
References
1. Landis, R. F.; Gupta, A.; Lee, Y. W.; Wang, L. S.; Golba, B.; Couillaud, B.; Ridolfo, R.; Das, R.; Rotello, V. M. ACS. Nano. 2017, 11, 1, 946-952. DOI: https://doi.org/10.1021/acsnano.6b07537.
2. Negi, A.; Kesari, K. K. Micromachines. 2022, 13, 8, 1265. DOI: https://doi.org/10.3390/mi13081265.
3. Saporito, F.; Sandri, G.; Bonferoni, M. C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Int. J. nanomedicine. 2018, 13, 8, 175-186. DOI: https://doi.org/10.2147/IJN.S152529.
4. Trifan, A.; Luca, S. V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A. C. Crit. Rev. Microbiol. 2020, 46, 3, 338-357. DOI: https://doi.org/10.1080/1040841X.2020.1782339.
5. Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Cancer. lett. 2014, 347, 2, 159-166. DOI: https://doi.org/10.1016/j.canlet.2014.03.013.
6. Seibert, J. B.; Viegas, J. S. R.; Almeida, T. C.; Amparo, T. R.; Rodrigues, I. V.; Lanza, J. S.; Frézard, F. J. G.; Soares, R.; Teixeira, L. F. M.; de Souza, G. H. B.; Vieira, P. M. A.; Barichello, J. M.; Dos Santos, O. D. H. J. Nat. Prod. 2019, 82, 12, 3208-3220. DOI: https://doi.org/10.1021/acs.jnatprod.8b00870.
7. Wu, H.; Moser, C.; Wang, H. Z.; Høiby, N.; Song, Z. J. Int. J. Oral. Sci. 2015, 7, 1, 1-7. DOI: https://doi.org/10.1038/ijos.2014.65.
8. Diniz do Nascimento, L.; Moraes, A. A. B.; Costa, K. S. D.; Pereira Galúcio, J. M.; Taube, P. S.; Costa, C. M. L.; Neves Cruz, J.; de Aguiar Andrade, E. H.; Faria, L. J. G. Biomolecules. 2020, 10, 7, 988. DOI: https://doi.org/10.3390/biom10070988.
9. Soltanzadeh, M.; Peighambardoust, S. H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J. M. Int. J. Biol. Macromol. 2021, 192, 1084-1097. DOI: https://doi.org/10.1016/j.ijbiomac.2021.10.070
10. Dong, N. N.; Chen, X. L.; Deng, B. L.; Xie, S. C.; Hu, J. J. Chin. Mater. Med. 2023, 48, 4,1076-1086. DOI: https://10.19540/j.cnki.cjcmm.20221102.703.
11. Moreira, P.; Matos, P.; Figueirinha, A.; Salgueiro, L.; Batista, M. T.; Branco, P. C.; Cruz, M.T.; Pereira, C. F. Int. J. Mol. Sci. 2022, 23, 15, 8812. DOI: https://doi.org/10.3390/ijms23158812.
12. Qin, T. T.; Xie, H. X.; Hu, J. W.; Zeng, J. S.; Liu, R.; Zeng, N. J. Chin. Mater. Med. 2023, 48, 4, 1066-1075. DOI: https://10.19540/j.cnki.cjcmm.20221014.705.
13. Silva, P.; Rodríguez-Pérez, M.; Gómez-Torres, Ó.; Burgos-Ramos, E. Nutr. Res. Rev. 2023, 36, 1, 140-154. DOI: https://doi.org/10.1017/S095442242100041X.
14. Luo, J. F.; Zhou, H.; Lio, C. K. Molecules(Basel,Switzerland). 2022, 27, 19, 6236. DOI: https://doi.org/10.3390/molecules27196236.
15. Xi, Y.; Zhao, T.; Shi, M.; Zhang, X.; Bao, Y.; Gao, J.; Shen, J.; Wang, H.; Xie, Z.; Wang, Q.; Li, Z.; Qin, D. Evid. Based. Complement. Alternat. Med. 2023, 2023, 2140327. DOI: https://doi.org/10.1155/2023/2140327.
16. Yao, W. L.; Pan, J.; Niu, T. F.; Yang, X. L.; Zhao, S. J.; Wang, Z. T.; Wang, R. F. Chin. Mater. Med. 2022, 47, 17, 4593-4599. DOI: https://10.7501/j.issn.0253-2670.2013.11.022.
17. Tian, S.; Zou, Y.; Wang, J.; Li, Y.; An, B. Z.; Liu, Y. Q. J. Ethnopharmacol. 2022, 295, 115399. DOI: https://doi.org/10.1016/j.jep.2022.115399.
18. Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Mehramuz, B.; Kafil, H. S. Colloids. Interface. Sci. Commun. 2018, 22, 18-24. DOI: https://doi.org/10.1016/j.colcom.2017.11.006.
19. Liu, B.; Kou, J.; Li, F.; Huo, D.; Xu, J.; Zhou, X.; Meng, D.; Ghulam, M.; Artyom, B.; Gao, X.; Ma, N.; Han, D. Aging. 2020, 12, 9, 8622-8639. DOI: https://doi.org/10.18632/aging.103179.
20. Cai, M.; Wang, Y.; Wang, R.; Li, M.; Zhang, W.; Yu, J.; Hua, R. Int. J. Biol. Macromol. 2022, 202, 122-129. DOI: https://doi.org/10.1016/j.ijbiomac.2022.01.066.
21. Miranda, M.; Cruz, M.T.; Vitorino, C.; Cabral, C. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 103, 109804. DOI: https://doi.org/10.1016/j.msec.2019.109804.
22. Tenchov, R.; Bird, R.; Curtz, A. E.; Zhou, Q. ACS. Nano. 2021, 15, 11, 16982-17015. DOI: https://doi.org/10.1021/acsnano.1c04996.
23. Wu, B.; Li, Y.; Li, Y. Y.;Shi, Z. H.; Bian, X. H.; Xia,Q. J. Biomater. Appl. 2022, 36, 8, 1444-1457. DOI: https://doi.org/10.1177/08853282211053923.
24. Bashiri, S.; Ghanbarzadeh, B.; Ayaseh, A.; Dehghannya, J.; Ehsani, A. LWT. 2020, 119, 108836. DOI: https://doi.org/10.1016/j.lwt.2019.108836
25. Yostawonkul, J.; Surassmo, S.; Iempridee, T.; Pimtong, W.; Suktham, K.; Sajomsang, W.; Gonil, P.; Ruktanonchai, U. R. Colloids. Surf. B. 2017, 149, 301-311. DOI: https://doi.org/10.1016/j.colsurfb.2016.09.049.
26. Wang, M.; Zhao, T.; Liu, Y.; Wang, Q.; Xing, S.; Li, L.; Wang, L.; Liu, L.; Gao, D.Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 71, 1231-1240. DOI: https://doi.org/10.1016/j.msec.2016.11.014.
27. Hasan, M.; Elkhoury, K.; Belhaj, N.; Kahn, C.; Tamayol, A.; Barberi-Heyob, M.; Arab-Tehrany, E.; Linder, M. Mar. Drugs. 2020, 18, 4, 217. DOI: https://doi.org/10.3390/md18040217.
28. Ramezanzade, L.; Hosseini, S. F.; Nikkhah, M. Food. Chem. 2017, 234, 220-229. DOI: https://doi.org/10.1016/j.foodchem.2017.04.177.
29. Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S.; Chang, D. Food. Funct. 2015, 6,12, 3702-3711. DOI: https://doi.org/10.1039/c5fo00256g.
30. Du, W.; Li, X.; Yang, Y.; Yue, X.; Jiang, D.; Ge, W.; Cai, B. Pharm. Biol. 2017, 55, 1, 2129-2135. DOI: https://doi.org/10.1080/13880209.2017.1297469.
31. Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Mar. Drugs. 2018, 16, 11, 432. DOI: https://doi.org/10.3390/md16110432
32. Ruan, N.; Jiao, Z.; Tang, L. J. AOAC. Int. 2022, 105, 1, 272-281. DOI: https://doi.org/10.1093/jaoacint/qsab108.
33. Soh, S. H.; Lee, L. Y. Pharmaceutics. 2019, 11, 1, 21. DOI: https://doi.org/10.3390/pharmaceutics11010021.
34. Kuś, P. M.; Okińczyc, P.; Jakovljević, M.; Jokić, S.; Jerković, I. J. Pharm. Biomed. Anal. 2018, 158, 15-27. DOI: https://doi.org/10.1016/j.jpba.2018.05.041.
35. Wang, J.; Wang, H.; Xu, H.; Li, J.; Zhang, X.; Zhang, X. RSC. Adv. 2022,12,11,6583-6591. DOI: https://doi.org/10.1039/d1ra07638h.
36. Yao, K.; Ma, Y. Z.; Yi, G. H.; Wang, Y. X.; Zhou, X.; Zang, X.; Liu, Y. Q.; Wang, T.; He, X.W. Chin. J. Trop. Crops. 2022, 44, 7, 1478-1487. DOI: https://doi.org/10.3969/j.issn.1000-2561.2023.07.019
37. Lee, S. A.; Joung, H. J.; Park, H. J.; Shin, G. H. J. Food. Sci. 2017, 82, 4, 904-912. DOI: https://doi.org/10.1111/1750-3841.13655.
38. Kumbhar, D. D.; Pokharkar, V. B. Colloid. Surface. A. 2013, 416, 32-42. DOI: https://doi.org/10.1016/j.colsurfa.2012.10.031.
39. outo, E. B.; Müller, R. H. J. Microencapsul. 2005, 22, 501-510. DOI: https://doi.org/10.1080/02652040500162436.
40. Keivani Nahr, F.; Ghanbarzadeh, B.; Hamishehkar, H.; Samadi Kafil, H. J. Funct. Foods. 2018, 40, 1-8. DOI: https://doi.org/10.1016/j.jff.2017.09.028.
41. Hu, Q.; Zhou, F.; Ly, N. K.; Ordyna, J.; Peterson, T.; Fan, Z.; Wang, S. ACS. Nano. 2023, 17, 8586-8597. DOI: https://doi.org/10.1021/acsnano.3c01094
42. Teeranachaideekul, V.; Boonme, P.; Souto, E. B.; Müller, R. H.; Junyaprasert, V. B. J. Control. Release. 2008, 128, 134-141. DOI: https://doi.org/10.1016/j.jconrel.2008.02.011.
43. Chaiyasan, W.; Srinivas, S. P.; Tiyaboonchai, W. Mol. Vis. 2015, 21, 1224-1134. DOI: https://10.1016/J.IJPHARM.2021.121287.
44. Sun, R.; Xia, Q. Colloid. Surface. A. 2019, 574, 197-206. DOI: https://doi.org/10.1016/j.colsurfa.2019.04.082.
45. Pardeike, J.; Hommoss, A.; Müller, R. H. Int. J. Pharm. 2009, 366, 170-184. DOI: https://10.1016/j.ijpharm.2008.10.003.
46. Rai, N.; Madni, A.; Faisal, A.; Jamshaid, T.; Khan, M. I.; Khan, M. M.; Parveen, F. Curr. Drug. Deliv. 2021, 18, 1368-1376. DOI: https://doi.org/10.2174/1567201818666210203180153.
47. Yui, T.; Uto, T.; Ogawa, K. Nanomaterials (Basel, Switzerland). 2021, 11, 6, 1407. DOI: https://doi.org/10.3390/nano11061407.
48. Averina, E. S.; Seewald, G.; Müller, R. H.; Radnaeva, L. D.; Popov, D. V. Pharmazie. 2010, 65, 1, 25-31. DOI: https://10.1691/ph.2010.9203.
49. Mendes, A. I.; Silva, A. C.; Catita, J. A.; Cerqueira, F.; Gabriel, C.; Lopes, C. Colloids. Surf. B. 2013, 111, 755-763. DOI: https://doi.org/10.1016/j.colsurfb.2013.05.041.
50. Hu, Q.; Lu, Y.; Luo, Y. Carbohydr. Polym. 2021, 264, 117999. DOI: https://doi.org/10.1016/j.carbpol.2021.117999.
51. Araújo, J.; Nikolic, S.; Egea, M.A.; Souto E.B.; Garcia M.L. Colloids and Surfaces B: Biointerfaces, 2011, 88, 150–157. DOI: https://doi.org/10.1016/j.colsurfb.2011.06.025.
52. Yang, Y.H.; Pan, H.Z.; Song, B.J.; Zhu, X.J.; Sun, Y.W. Prog. Modern. Biomed. 2011, 11, 4024-4026. DOI: 10.13241/j.cnki.pmb.2011.21.006.

Downloads
Published
Issue
Section
License
Copyright (c) 2025 Yu Zhang, Mengjiao Mengjiao, Jing Zou, Chaojun Li, Ming Kang, Xiaofeng Liang

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








