Electrochemical and Gravimetric Study on Corrosion Inhibition of Carbon Steels Exposed to Oilfield Produced Water
DOI:
https://doi.org/10.29356/jmcs.v67i4.1937Keywords:
Inhibitor, Pipeline steel, Protection, Storage tanksAbstract
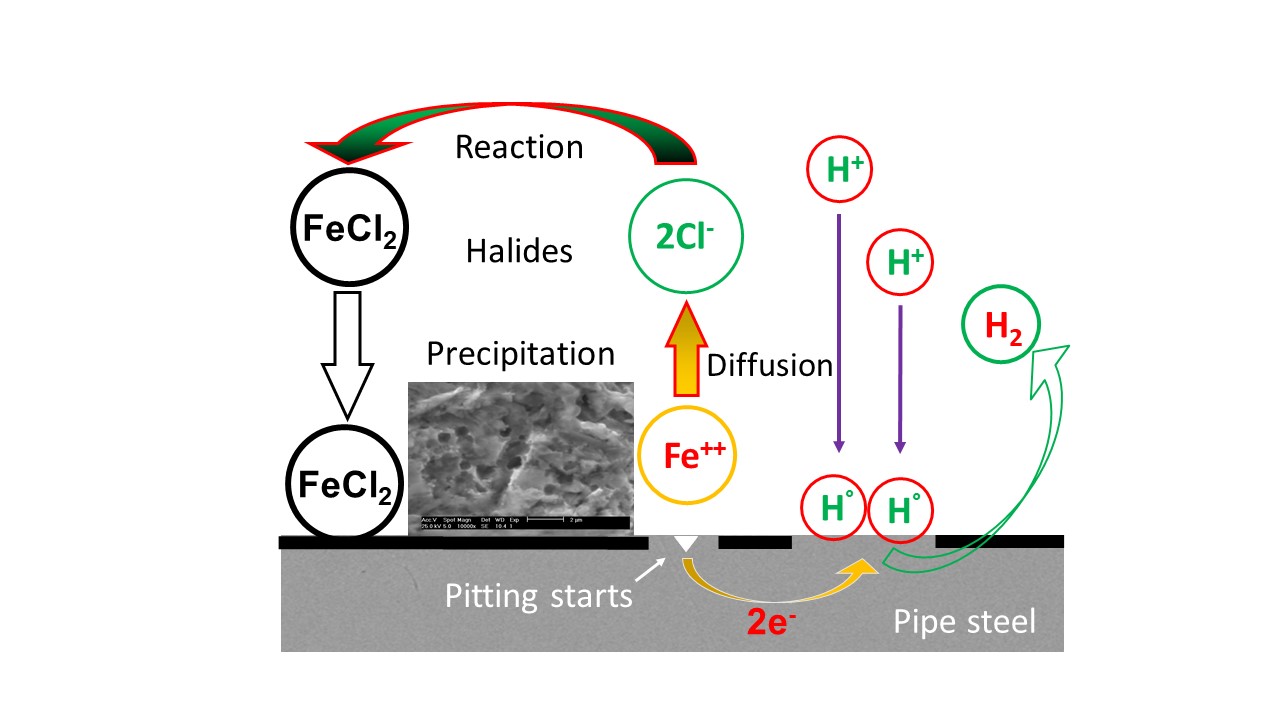
Abstract. Two corrosion inhibitors (CI) were evaluated to study the protection behaviours of three carbon steels: X52, X60, and X70 in an oilfield produced water. The water was subjected to unstirred condition and a rotation speed of 600 rpm to simulate a stagnant and homogeneous solutions, respectively, it is in pipelines at temperature range of 30 °C to 60 °C. The internal corrosion rate and inhibition efficiencies were measured using polarization curves and gravimetric tests, complimented with the surface analysis of the corroded carbon steel samples using scanning electron microscopy (SEM). The experimental results suggested that the chlorides compounds, H2S, metals, and the inhibitor type modified the corrosion rate of the carbon steels under study. High corrosion rates were achieved on X70 steel at the temperature of 30 °C and 50 °C under 600 rpm. It was determined that X52 steel had the highest corrosion rate at 60 °C and 600 rpm. While an adequate protection of X70 steel was confirmed with a high inhibition efficiency using a naphthenic imidazoline as corrosion inhibitor.
Resumen. Se evaluaron dos inhibidores de corrosión para estudiar los comportamientos de protección de tres aceros al carbono: X52, X60 y X70 en agua congénita. El agua se sometió a condiciones sin agitación y una velocidad de rotación de 600 rpm para simular soluciones estancadas y homogéneas, respectivamente, el cual se encuentra en tanques de almacenamiento y tuberías en un rango de temperatura de 30 °C a 60 °C. La velocidad de corrosión interna y los valores de las eficiencias a la inhibición se determinaron mediante curvas de polarización y pruebas gravimétricas, las que fueron complementadas con el análisis de la superficie de las muestras de acero al carbono corroídas mediante microscopía electrónica de barrido. Los resultados experimentales sugirieron que los compuestos de cloruros, H2S, metales y el tipo de inhibidor, modificaron la velocidad de corrosión de los aceros al carbono en estudio. Altos valores de corrosión en acero X70 fueron alcanzados a la temperatura de 30 °C y 50 °C usando 600 rpm. Se determinó que el acero X52 tuvo la velocidad de corrosión más alta a 60 °C y 600 rpm. Mientras que se confirmó una protección adecuada del acero X70 con una alta eficiencia de inhibición usando imidazolina nafténica como inhibidor de corrosión.
Downloads
References
Groysman, A. Czech Assoc. Corros. Eng. 2017, 61, 100-117. DOI: https://doi.org/10.1515/kom-2017-0013
Lahme, S.; Mand, J.; Longwell, J.; Smith, R.; Enning, D. Appl. Environ. Microbiol. 2021, 87, 1-17. DOI: https://doi.org/10.1128/AEM.01819-20
Foroulis, Z. A. Anti-Corros. Methods Mater. 1981, 28, 4-9. DOI: https://doi.org/10.1108/eb007174
Fouda, A. S.; Elmorsi, M. A.; Fayed, T.; Shaban, S.M.; Azazy, O. Surf. Eng. Appl. Electrochem. 2018, 54, 180-193. DOI: https://doi.org/10.3103/S1068375518020060
Shao, Y.; Tang, J.; Zhang, T.; Meng, G.; Wang, F. Int. J. Electrochem. Sci. 2013, 8, 5546-5559. DOI: https://doi.org/10.1016/S1452-3981(23)14703-1
Álvarez-Manzano, R.; Mendoza-Canales, J.; Castillo-Cervantes, S.; Marín-Jesús, J. J. Mex. Chem. Soc. 2013, 57, 30-35.
Garnica-Rodríguez, A.; Genesca, J.; Mendoza-Flores, J.; Duran-Romero, R. J. Appli. Electrochem. 2009, 39, 1809-1819. DOI: https://doi.org/10.1007/s10800-009-9884-4
Xuejun, X.; Wanqin, Y.; Shunan, C.; Ling, P.; Xunjie, G.; Keru, P. Anti-Corrosos. Methods Mater. 2005, 52, 145-147. DOI: https://doi.org/10.1108/00035590510595120
Liu, D.; Chen, Z. Y.; Guo, X. P. Anti-Corrosos. Methods Mater. 2008, 55, 130-134. DOI: https://doi.org/10.1108/00035590810870437
Cheng, Y.; Bai, Y.; Li, Z.; Liu, J. G. Anti-Corrosos. Methods Mater. 2019, 66, 174-187. DOI: https://doi.org/10.1108/ACMM-07-2018-1969
Zhou, C.; Huang, Q.; Gou, Q.; Zheng, J.; Chen, X. Corros. Sci. 2016, 110, 242-252. DOI: https://doi.org/10.1016/j.corsci.2016.04.044
Lu, Y.; Zhang, Y.; Liu, Z.; Zhang, Y.; Wang, C.; Guo, H. Anti-Corrosos. Methods Mater. 2014, 61, 166-171. DOI: https://doi.org/10.1108/ACMM-02-2013-1240
Zafar, M.N.; Rihan, R.; Al-Hadhrami, L. Corros. Sci. 2015, 94, 275-287. DOI: https://doi.org/10.1016/j.corsci.2015.02.013
Demoz, A.; Papavinasam, S.; Omotoso, O.; Michaelian, K.; Revie, R. W. Corrosion. 2009, 65, 741-747. DOI: https://doi.org/10.5006/1.3319100
Liao, K.; Yao, Q.; Wu, X.; Jia, W. Energies. 2012, 5, 3892-3907. DOI: https://doi.org/10.3390/en5103892
Bandyopadhyay, S.; Neogy, C.; Den, S. K. Phys. Stat. Sol. 1984, 122, 113-123. DOI: https://doi.org/10.1002/pssb.2221220113
Refaey, S. A. M.; Taha, F.; Abd El-Malak, A. M. Int. J. Electrochem. Sci. 2006, 1, 80-91. DOI: https://doi.org/10.1016/S1452-3981(23)17138-0
Zarrok, H.; Zarrouk, A.; Salghi, R.; Ebn Touhami, M.; Oudda, H.; Hammouti, B.; Touir, R.; Bentis, F.; Al-Deyab, S. S. Int. J. Electrochem. Sci. 2013, 8, 6014-6032. DOI: https://doi.org/10.1016/S1452-3981(23)14736-5
Quej Ake, L. M.; Contreras, A.; Liu, H. B.; Alamilla, J. L.; Sosa, E. S.E.A.E. 2020, 56, 365-380. DOI: https://doi.org/10.3103/S1068375520030126
Quej Ake, L. M.; Contreras, A.; Liu, H. B.; Alamilla, J. L.; Sosa, E. Anti-Corrosos. Methods Mater. 2018, 65, 281-291. DOI: https://doi.org/10.1108/ACMM-12-2017-1874
Quej-Aké, L. M.; Contreras, A.; Aburto, J., in: Characterization of Metals and Alloys, Vol. 1, Pérez Campos, R.; Contreras Cuevas, A.; Esparza Muños, R.A., Eds., Springer, Switzerland, 2017, 13-28.
Quej-Aké, L. M.; Mireles, M. J.; Galvan-Martínez, R.; Contreras, A., in: Materials Characterization, Vol. 1, Pérez Campos, R.; Contreras Cuevas, A.; Esparza Muños, R.A., Eds., Springer, Switzerland, 2015, 101-118.
Quej Ake, L.M.; Contreras, A.; Aburto, J. Int. J. Electrochem. Sci. 2018, 13, 7416-7431. DOI: https://doi.org/10.20964/2018.08.73
Bard A.J.; Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications. J. Wiley & Sons, New York, 1980.
EPA 6010C. Inductively coupled plasma-atomic emission spectrometry (ICP-AES), 2000.
ASTM D4327-17. Standard Test Method for Anions in Water by Chemically Suppressed Ion Chromatography, ASTM International, West Conshohocken, PA, 2017.
Benítez, J. L.; Guzmán, G.; Muñoz L.; Sánchez, L. O.G.J. 2004, May 24, 60-65.
Benítez, J. L.; Martínez, C.; Roldan, R. O.G.J. 2002, June 17, 66-70.
Quej-Ake, L.M.; Marín Cruz, J.; Contreras, A. Anti-Corrosos. Methods Mater. 2017, 64, 61-68. DOI: https://doi.org/10.1108/ACMM-03-2015-1512
ASTM G170. Standard Guide for Evaluating and Qualifying Oilfield and Refinery Corrosion Inhibitors in the Laboratory, ASTM International, West Conshohocken, PA, 2012.
ASTM G184. Standard Test Method for Evaluating and Qualifying Oil and Refinery Corrosion Inhibitors Using Rotating Cage, ASTM International, West Conshohocken, PA, 2016.
ASTM G185. Standard Test Method for Evaluating and Qualifying Oil Field and Refinery Corrosion Inhibitors Using the Rotating Cylinder Electrode, ASTM International, West Conshohocken, PA, 2016.
NACE Standard TM0177. Laboratory Testing of Metals for Resistance to Specific Forms of Environmental Cracking in H2S Environments, NACE International, TX, US, 2005.
Vedage, H.; Ramanarayanan, T. A.; Mumford J.D.; Smith, S. N. Corrosion. 1993, 49, 114-121. DOI: https://doi.org/10.5006/1.3299205
ASTM G102. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements, ASTM International, West Conshohocken, PA, 2015.
El Mehdi, B.; Mernari, B.; Traisnel, M.; Bentiss, F.; Lagrenée, M. Corros. Sci. 2002, 77, 489-496. DOI: https://doi.org/10.1016/S0254-0584(02)00085-8
NACE Standard SP0775. Preparation, Installation, Analysis, an Interpretation of Corrosion Coupons in Oilfield Operations, NACE International, TX, US, 2018.
ASTM G1-03(17). Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens, ASTM International, West Conshohocken, PA, 2017.
NACE/ASTM TM0169. Laboratory Corrosion Testing of Metals, NACE International, TX, US, 2012.
Zhang, G. A.; Cheng, Y.F. Corros. Sci. 2009, 51, 87-94. DOI: https://doi.org/10.1016/j.corsci.2008.10.013
Sherif, E. S. M.; Almajid, A. A.; Khalil, A. K.; Junaedi, H.; Latief, F.H. Int. J. Electrochem. Sci. 2013, 9360-9370. DOI: https://doi.org/10.1016/S1452-3981(23)12975-0
Peng, H.; Luan, Z.; Liu, J.; Lei, Y.; Chen, J.; Deng, S.; Su, X. Anti-Corrosos. Methods Mater. 2021, 68, 438-448. DOI: https://doi.org/10.1108/ACMM-06-2020-2328
Quej Ake, L. M.; Contreras, A.; Lui H.; Alamilla, J. L.; Sosa, E. Anti-Corrosos. Methods Mater. 2019, 66, 101-114. DOI: https://doi.org/10.1108/ACMM-06-2018-1959
Quej Ake, L. M.; Contreras, A.; Aburto, J. E. Anti-Corrosos. Methods Mater. 2018, 65, 234-248. DOI: https://doi.org/10.1108/ACMM-03-2017-1770
Bentiss, F.; Lebrini, M.; Lagrenée, M. Corros. Sci. 2005, 47, 2915-2931. DOI: https://doi.org/10.1016/j.corsci.2005.05.034
Negm, N. A.; Al Sabagh, A. M.; Migahed, M. A.; Abdel Bary, H. M.; El Din, H. M. Corros. Sci. 2010, 52, 2122-2132. DOI: https://doi.org/10.1016/j.corsci.2010.02.044
Arcenegui Troya, J.; Sánchez Jiménez, P.E.; Perejón, A.; Pérez Maqueda, L. A. J. Therm. Anal. Calorim. 2022. DOI: https://doi.org/10.1007/s10973-022-11728-3.
Seikh, A. H.; Sherif, E. S. M. Int. J. Electrochem. Sci. 2015, 895-908. DOI: https://doi.org/10.1016/S1452-3981(23)05042-3
Noor El-Din, M. R.; Al Sabagh, A. M.; Hegazy, E. M. A. J. Dispersion Sci. Technol. 2012, 33, 1444-1451. DOI: https://doi.org/10.1080/01932691.2011.605660
Gogoi, P. K.; Barhai, B. Int. J. Electrochem. Sci. 2011, 136-145. DOI: https://doi.org/10.1016/S1452-3981(23)14981-9
Li, S.; Zeng, Z.; Harris, M. A.; Sánchez, L. J.; Cong, H. Frontiers in Maters. 2019, 6, 10.
DOI: 10.3389/fmats.2019.00010. DOI: https://doi.org/10.3389/fmats.2019.00010
Quej Ake, L. M.; García Jiménez, S.; Liu, H. B.; Alamilla, J. L.; Angeles Chavez, C. Anti-Corrosos. Methods Mater. 2022, 69, 131-140. DOI: https://doi.org/10.1108/ACMM-06-2021-2492
ASTM G5. Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements, ASTM International, West Conshohocken, PA, 2021.

Downloads
Published
Issue
Section
License
Copyright (c) 2023 Luis Quej Ake, J. L. Alamilla, A. Contreras

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









