Impact of the Chemical Speciation of the Ag⁺–Cl⁻–e⁻ System on the Construction of True Reference Electrodes and the Potential Purification of the Ionic Liquid 1-Butyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide
DOI:
https://doi.org/10.29356/jmcs.v69i4.2374Keywords:
Room temperature ionic liquids, reference electrodes, silver, chemical speciation, liquid-liquid extractionAbstract
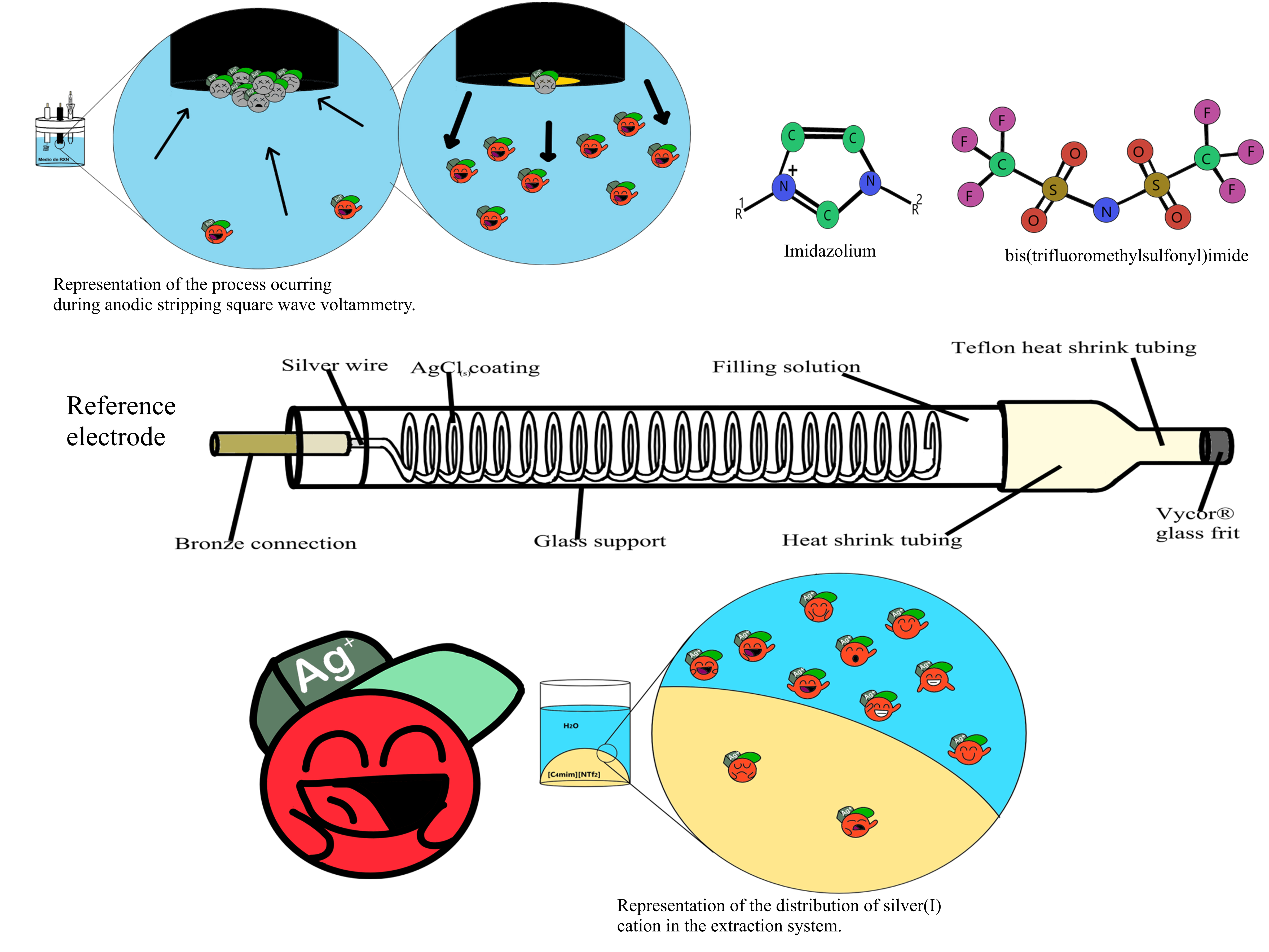
Abstract. We present the chemical speciation of [AgCln]1-n/Ag0 redox couple in two media: the room temperature ionic liquid (RTIL) 1‑butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C4mim][NTf2]) as a model ionic solvent, and aqueous medium. The logarithms of the formation constants (log βn), solubility product constant (pKsp), and formal reduction potential (E°') values of these chemical systems were estimated through open circuit potential measurements using suitable indicator electrodes and representative potentiometric titrations. The estimation of the extraction constant (KE) of Ag+ in the interphase water-RTIL was determined through a series of extraction systems at different values of p(Vorg/Vac), finding that the extraction of silver(I) is favorable towards aqueous media at high pCl values. Also, a series of reference electrodes (RE) were constructed under different buffer conditions for use in this ionic liquid to assess the utility of the collected electrochemical data. The potential drift of the half-cells was determined via cyclic voltammetry using the cobaltocene redox couple, [Co(Cp)2]+/0, as an internal redox reference; in addition, information on the Ag(I) extraction constant allowed to explain the effect of water as a contaminant of these devices. Finally, specific configurations were identified for these REs, some exhibiting potential drifts of less than 0.58 μV h-1, rendering them comparable to commonly used REs in aqueous media.
Resumen. Se presenta la especiación química del par redox [AgCln]1-n/Ag0 en dos medios: el líquido iónico a temperatura ambiente (RTIL) bis(trifluorometilsulfonil)imida de 1-butil-3-metilimidazolio ([C4mim][NTf2]) como solvente iónico modelo, y en medio acuoso. Los logaritmos de las constantes de formación (log βn), la constante del producto de solubilidad (pKps) y los valores del potencial formal de reducción (E°’) de estos sistemas químicos se estimaron mediante mediciones de potencial de circuito abierto utilizando electrodos indicadores adecuados y valoraciones potenciométricas representativas. La estimación de la constante de extracción (KE) de Ag⁺ en la interfase agua-RTIL se determinó a través de una serie de sistemas de extracción a diferentes valores de p(Vorg/Vac), encontrando que la extracción de plata(I) es favorable hacia el medio acuoso a altos valores de pCl. Además, se construyó una serie de electrodos de referencia (RE) bajo diferentes condiciones de amortiguamiento para su uso en este líquido iónico, con el fin de evaluar la utilidad de los datos electroquímicos obtenidos. La deriva potencial de las semiceldas se determinó mediante voltamperometría cíclica usando el par redox de cobaltoceno, [Co(Cp)2]+/0, como referencia redox interna además, la información sobre la constante de extracción de Ag(I) permitió explicar el efecto del agua como contaminante de estos dispositivos. Finalmente, se identificaron configuraciones específicas para estos REs, algunos de los cuales exhibieron derivas potenciales de menos de 0.58 μV h⁻¹, haciéndolos comparables con los REs comúnmente utilizados en medios acuosos.
Downloads
References
1. Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D. R. Chem. Rev. 2017, 117, 6633–6635. DOI: https://doi.org/10.1021/acs.chemrev.7b00246.
2. Wasserscheid, P.; Welton, T., in: Ionic Liquids in Synthesis, 2nd ed.; Wiley, 2008; Vol. 1. DOI: 10.1002/9783527621194. DOI: https://doi.org/10.1002/9783527621194
3. Barrosse-Antle, L. E.; Bond, A. M.; Compton, R. G.; O’Mahony, A. M.; Rogers, E. I.; Silvester, D. S. Chem. Asian J. 2010, 5, 202–230. DOI: https://doi.org/10.1002/asia.200900191.
4. Caminiti, R.; Gontrani, L., in: The Structure of Ionic Liquids, 1st ed.; Springer, 2013. DOI: https://doi.org/10.1007/978-3-319-01698-6.
5. Tiago, G. A. O.; Matias, I. A. S.; Ribeiro, A. P. C.; Martins, L. M. D. R. S. Molecules. 2020, 25, 5812. DOI: https://doi.org/10.3390/molecules25245812.
6. Cruz, C.; Ciach, A. Molecules. 2021, 26, 3668. DOI: https://doi.org/10.3390/molecules26123668.
7. Piatti, E.; Guglielmero, L.; Tofani, G.; Mezzetta, A.; Guazzelli, L.; D’Andrea, F.; Roddaro, S.; Pomelli, C. S. J. Mol. Liq. 2022, 364, 120001. DOI: https://doi.org/10.1016/j.molliq.2022.120001.
8. Ratti, R. Adv. Chem. 2014, 1–16. DOI: https://doi.org/10.1155/2014/729842.
9. Riaño, S. Ionic Liquids as Green Solvents: A Critical Analysis, in: Encyclopedia of Green Chemistry; Elsevier, 2025; 43–53. DOI: https://doi.org/10.1016/b978-0-443-15742-4.00019-3.
10. Gong, K.; Fang, Q.; Gu, S.; Li, S. F. Y.; Yan, Y. Energy Environ. Sci. 2015, 8, 3515–3530. DOI: https://doi.org/10.1039/c5ee02341f.
11. Torriero, A. A. J., in: Electrochemistry in Ionic Liquids. Volume 1: Fundamentals; Springer, 2015; Vol. 1. DOI: https://doi.org/10.1007/978-3-319-13485-7. DOI: https://doi.org/10.1007/978-3-319-13485-7_1
12. Snook, G. A.; Best, A. S.; Pandolfo, A. G.; Hollenkamp, A. F. Electrochem. commun. 2006, 8, 1405–1411. DOI: https://doi.org/10.1016/j.elecom.2006.07.004.
13. Huber, B.; Roling, B. Electrochim. Acta. 2011, 56, 6569–6572. DOI: https://doi.org/10.1016/j.electacta.2011.02.055.
14. Horwood, C.; Stadermann, M. Electrochem. Commun. 2018, 88, 105–108. DOI: https://doi.org/10.1016/j.elecom.2018.02.005.
15. García-Mendoza, A.; Aguilar-Cordero, J. C. Electrochim. Acta. 2019, 302, 344–351. DOI: https://doi.org/10.1016/j.electacta.2019.02.029.
16. Nevell, T. G.; Walsh, F. C. Trans. IMF 1992, 70, 144–147. DOI: https://doi.org/10.1080/00202967.1992.11870962.
17. Inzelt, G.; Lewenstam, A.; Scholz, F., in: Handbook of Reference Electrodes; Springer, 2013. DOI: https://doi.org/10.1007/978-3-642-36188-3_6.
18. Bard, A. J.; Faulkner, L. R.; White, H. S. Electrochemical Methods - Fundamentals and Applications, 3rd ed.; Wiley, 2022. DOI: https://doi.org/10.1007/s11243-023-00555-6.
19. Bard, A. J., Inzelt, G., Scholz, F., in: Electrochemical Dictionary; Springer, 2008. DOI: https://doi.org/10.1007/978-3-540-74598-3.
20. Sukardi, S. K.; Zhang, J.; Burgar, I.; Horne, M. D.; Hollenkamp, A. F.; MacFarlane, D. R.; Bond, A. M. Electrochem. commun. 2008, 10, 250–254. DOI: https://doi.org/10.1016/j.elecom.2007.11.022.
21. Scholz, F., in: Electroanalytical Methods, Guide to Experiments and Applications; Springer, 2010. DOI: https://doi.org/10.1007/978-3-642-02915-8.
22. Inzelt, G.; Lewenstam, A.; Scholz, F., in: Handbook of Reference Electrodes; Springer, 2013. DOI: https://doi.org/10.1007/978-3-642-36188-3_6.
23. Lockett, V.; Horne, M.; Sedev, R.; Rodopoulos, T.; Ralston, J. Phys. Chem. Chem. Phys. 2010, 12 , 12499–12512. DOI: https://doi.org/10.1039/c0cp00170h.
24. Fedorov, M. V.; Kornyshev, A. A.; Georgi, N. Electrochem. commun. 2010, 12, 296–299. DOI: https://doi.org/10.1016/j.elecom.2009.12.019.
25. Yalcinkaya, F.; Powner, E. T. Méd. Eng. Phys. 1997, 19, 299–301. DOI: https://doi.org/10.1016/s1350-4533(96)00034-3.
26. Fleischmann, M.; Hiddleston, J. N. J. Phys. E: Sci. Instrum. 2002, 1, 667. DOI: https://doi.org/10.1088/0022-3735/1/6/424.
27. Martell, A. E.; Smith, R. M., in: Critical Stability Constants. Volume 4: Inorganic Complexes; Springer, 1976; Vol. 4. DOI: https://doi.org/10.1007/978-1-4757-5506-0.
28. Rodil, E.; Aldous, L.; Hardacre, C.; Lagunas, M. C. Nanotechnology 2008, 19, 105603–105608. DOI: https://doi.org/10.1088/0957-4484/19/10/105603.
29. Martell, A. E.; Smith, R. M., in: Critical Stability Constants. Volume 6: Second Supplement; Springer, 1989; Vol. 6. DOI: https://doi.org/10.1007/978-1-4615-6764-6.
30. Gran, G. Acta Chem. Scand. 1950, 4, 559–577. DOI: https://doi.org/10.3891/acta.chem.scand.04-0559.
31. Brown, A. M. Comput. Methods Programs Biomed. 2001, 65, 191–200. DOI: https://doi.org/10.1016/S0169-2607(00)00124-3.
32. Briones-Guerash-S. U.; García-Mendoza, A.; Aguilar-Cordero, J. C. J. Chem. Educ. 2023, 100, 4663–4673. DOI: https://doi.org/10.1021/acs.jchemed.3c00790.
33. Davies, C. W.; Jones, A. L. Discuss. Faraday Soc. 1949, 5, 103–111. DOI: https://doi.org/10.1039/df9490500103.
34. Kim, J. I.; Duschner, H. J. Inorg. Nucl. Chem. 1977, 39, 4771–4478. DOI: https://doi.org/10.1016/0022-1902(77)80065-1
35. Dilleen, J. W.; Sprules, S. D.; Birch, B. J.; Haggett, B. G. D. The Analyst 1998, 123, 2905–2907. DOI: https://doi.org/10.1039/a806344c.
36. Johansson, P.-A.; Karlberg, B.; Thelander, S. Anal. Chim. Acta 1980, 114, 215–226. DOI: https://doi.org/10.1016/s0003-2670(01)84293-8.
37. Gavazov, K. B. Acta Chim. Slov. 2012, 59, 1–17.
38. Fritz, J. J. J. Solut. Chem. 1985, 14, 865–879. DOI: https://doi.org/10.1007/bf00646296.
39. Lloyd, P. J. D., in: Solvent Extraction Principles and Practice, Revised and Expanded, 2nd ed.; Marcel Dekker, 2004. DOI: https://doi.org/10.1201/9780203021460-14.
40. Ma, L.; Zhong, Z.; Hu, J.; Qing, L.; Jiang, J. J. Phys. Chem. B 2023, 127, 5308–5316. DOI: https://doi.org/10.1021/acs.jpcb.3c01559.
41. Tokuda, H.; Tsuzuki, S.; Susan, M. A. B. H.; Hayamizu, K.; Watanabe, M. J. Phys. Chem. B 2006, 110, 19593–19600. DOI: https://doi.org/10.1021/jp064159v.
42. Gebbie, M. A.; Smith, A. M.; Dobbs, H. A.; Lee, A. A.; Warr, G. G.; Banquy, X.; Valtiner, M.; Rutland, M. W.; Israelachvili, J. N.; Perkin, S.; Atkin, R. Chem. Commun. 2016, 53, 1214–1224. DOI: https://doi.org/10.1039/c6cc08820a.
43. Nordness, O.; Brennecke, J. F. Chem. Rev. 2020, 120, 12873–12902. DOI: https://doi.org/10.1021/acs.chemrev.0c00373.
44. Yalcinkaya, F.; Powner, E. T. Med. Eng. Phys. 1997, 19, 299–301. DOI: https://doi.org/10.1016/s1350-4533(96)00034-3.
45. Mousavi, M. P. S.; Saba, S. A.; Anderson, E. L.; Hillmyer, M. A.; Bühlmann, P. Anal. Chem. 2016, 88 (17), 8706–8713. DOI: https://doi.org/10.1021/acs.analchem.6b02025.
46. Vranes, M.; Dozic, S.; Djeric, V.; Gadzuric, S. J. Chem. Eng. Data 2012, 57, 1072–1077. DOI: https://doi.org/10.1021/je2010837.
47. Zoski, C. G., in: Handbook of Electrochemistry; Oxford, 2006.
48. García-Mendoza, A.; Aguilar, J. C. Electrochim. Acta. 2015, 182, 238–246. DOI: https://doi.org/10.1016/j.electacta.2015.09.045.

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2025 Jorge Ruvalcaba-Juárez, Oscar Valenzuela-Bonilla, Norma Rodríguez-Laguna, Arturo Garcia-Mendoza

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









