Bioreduction of the Chalcones by Fungus Scedosporium apiospermum EJCP13
DOI:
https://doi.org/10.29356/jmcs.v65i2.1454Keywords:
Biotransformation, bioreduction, chalcones, Scedosporium apiospermumAbstract
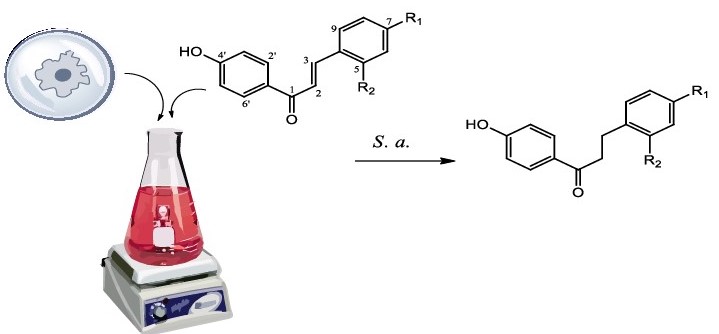
Abstract. Biotransformations are chemical reactions carried out by microorganisms on organic substrates. Biotransformations can be regio-, chemo-, stereo- and enantio-selective. Bioreductions are of great interest to the food and pharmaceutical industries as they help to reduce costs and impacts on the environment. In this work, the following biotransformations of chalcones were performed: (2E)-1-(4-hydroxy-phenyl)-3-(2-methoxy-phenyl)-prop-2-en-1-one (1), (2E)-1-(4-hydroxy-phenyl)-3-(4-methoxy-phenyl)-prop-2-en-1-one (2), and (2E)-1-(4-hydroxy-phenyl)-3-phenyl-prop-2-en-1-one (3) by the fungus Scedosporium aspiospermum, leading to formation through chemo-selective reduction of dihydrochalcones 1-(4-hydroxy-phenyl)-3-(2-methoxy-phenyl)-propan-1-one (4), 1-(4-hydroxy-phenyl)-3-(4-methoxy-phenyl)-propan-1-one (5), and 1-(4-hydroxy-phenyl)-3-phenyl-propan-1-one (6). Compounds 1-6 had their antimicrobial activities tested and were observed better activity to the biotransformation products compared with substrates. This is the first report of chemo-selective bioreduction by fungi of the genus Scedosporium in biotransformation reactions.
Resumen. Las biotransformaciones son reacciones químicas llevadas a cabo por microorganismos sobre sustratos orgánicos. Las biotransformaciones pueden ser regio-, quimio-, estereo- y enantio-selectivas. Las biorreducciones son de gran interés para la industria alimentaria y farmacéutica, ya que ayudan a reducir costes e impactos sobre el medio ambiente. En este trabajo se realizaron las siguientes biotransformaciones de las chalconas: (2E)-1-(4-hidroxi-fenil)-3-(2-metoxi-fenil)-prop-2-en-1-ona (1), ( 2E)-1-(4-hidroxi-fenil)-3-(4-metoxi-fenil)-prop-2-en-1-ona (2) y (2E)-1-(4-hidroxi-fenil)-3-fenil-prop-2-en-1-ona (3) por el hongo Scedosporium aspiospermum, que conduce a la formación mediante reducción quimio-selectiva de dihidrocalconas 1-(4-hidroxi-fenil)-3-(2-metoxi-fenil)-propan-1-ona (4), 1-(4-hidroxi-fenil)-3-(4-metoxi-fenil)-propan-1-ona (5) y 1-(4-hidroxi-fenil)-3-fenil-propan-1-ona (6). Se ensayaron las actividades antimicrobianas de los compuestos 1-6 y se observó una mejor actividad para los productos de biotransformación en comparación con los sustratos. Este es el primer informe de biorreducción quimio-selectiva por hongos del género Scedosporium en reacciones de biotransformación.
Downloads
References
Liu, J.; Yu, B. Curr. Org. Chem. 2010, 14, 1400-1406. DOI: https://doi.org/10.2174/138527210791616786
Perkins, C.; Siddique, S.; Puri, M.; Demain, A. L. Crit. Rev. Biotechnol. 2016, 36, 1050-1065 DOI: https://doi.org/10.3109/07388551.2015.1083943
Vasconcelos, D. H. P.; Mafezoli, J.; Uchôa, P. K. S.; Saraiva, N. N.; Lima, M. A. S.; Silva-Júnior, J. N.; Barbosa, F. G.; Mattos, M. C.; Oliveira, M. C. F.; Lima, C. S.; Pessoa, M. N. G. J. Braz. Chem. Soc. 2015, 26, 1043-1047 DOI: http://dx.doi.org/10.5935/0103-5053.20150070 DOI: https://doi.org/10.5935/0103-5053.20150070
Martins, L. R. M.; Takahashi, J. A. Nat. Prod. Res. 2010, 24, 767-774 DOI: http://dx.doi.org/10.1080/14786410903262865 DOI: https://doi.org/10.1080/14786410903262865
Cao, H.; Chen, X.; Jassbi, A. R.; Xiao, J. Biotechnol. Adv. 2015, 33, 214-223 DOI: https://doi.org/10.1016/j.biotechadv.2014.10.012
Alarcón, J.; Alderete, J.; Escobar, C.; Araya, R.; Cespedes, C. L. Biocatal. Biotransformation. 2013, 31, 160-167 DOI: https://doi.org/10.3109/10242422.2013.813489
Pathan, N. B.; Rahatgaonkar, A. M.; Chorghade, M. S. Indian J. Chem. 2012, 51, 992-1001.
Sanchez-Gonzalez, M.; Rosazza, J. P. N. J. Nat. Prod. 2004, 67, 553-558 DOI: https://doi.org/10.1021/np030448o
Botta, B.; Monache, G. D.; Rosa, M. C.; Scurria, R.; Vitali, A.; Vinciguerra, V.; Menendez, P.; Misiti, D. Heterocycles. 1996, 43, 1415-1421. DOI: https://doi.org/10.3987/COM-96-7414
Winska, K.; Grabarczyk, M.; Maczka, W.; Zarowska, B.; Maciejewska, G.; Dancewicz, K.; Gabrys, B.; Aniol, M. Appl. Sci. 2017, 7, 12 DOI: https://doi.org/10.3390/app7010012
Paduch, R,; Trytek, M.; Król, S. K.; Kud, J.; Frant, M.; Kandefer-Szersze?, M.; Fiedurek, J. Pharm. Biol. 2016, 54, 1096-1107 DOI: https://doi.org/10.3109/13880209.2015.1103753
Díaz-Tielas, C.; Graña, E.; Reigosa, M. J.; Sánchez-Moreiras, A. M. Planta Daninha 2016, 34, 607-616 DOI: https://doi.org/10.1590/s0100-83582016340300022
Cordeiro, J. S.; Carvalho, J. M.; Feitosa, A. O.; Pinheiro, E. A. A.; Marinho, P. S. B.; Marinho, A. M. R. Rev. Virtual Quim. 2019, 11, 210-2017 DOI: https://doi.org/10.21577/1984-6835.20190015
Corrêa, M. J. C.; Nunes, F. M.; Bitencourt, H. R.; Borges, F. C.; Guilhon, G. M. S. P.; Arruda, M. S. P.; Marinho, A. M. R.; Santos, A. S.; Alves, C. N.; Brasil, D. S. B.; Santos, L. S. J. Braz. Chem. Soc. 2011, 22, 1333-1338. DOI: https://doi.org/10.1590/S0103-50532011000700019
Pinheiro, E. A. A.; Pina, J. R. S.; Feitosa, A. O.; Carvalho, J. M.; Borges, F. C.; Marinho, P. S. B.; Marinho, A. M. R. Rev. Argent. Microbiol. 2017, 49, 3-6 DOI: http://dx.doi.org/10.1016/j.ram.2016.08.005 DOI: https://doi.org/10.1016/j.ram.2016.08.005
Tamilvanan, M.; Pandurangan, A.; Subramanian, K.; Reddy, B. S. R. Polym. Adv. Technol. 2008, 19, 1218-1225. DOI: https://doi.org/10.1002/pat.1114
Bowman, M. D.; Jacobson, M. M.; Pujanauski, B. G.; Blackwell, H. E. Tetrahedron. 2006, 62, 4715-4727. DOI: https://doi.org/10.1016/j.tet.2006.02.083
Karki, R.; Thapa, P.; Kang, M. J.; Jeong, T. C.; Nam, J. M.; Kim, H.; Na, Y.; Cho, W.; Kwon, Y.; Lee, E. Bioorg. Med. Chem. 2010, 18, 3066-3077 DOI: http://dx.doi.org/10.1016/j.bmc.2010.03.051 DOI: https://doi.org/10.1016/j.bmc.2010.03.051
Smith, H. M.; Knox, A. J. S.; Zisterer, D. M.; Lloyd, D. G.; Meegan, M. J. Medicinal Chemistry. 2007, 3, 135-155. DOI: https://doi.org/10.2174/157340607780059503
Colbon, P.; Ruan, J.; Purdie, M.; Xiao, J. Org. Lett. 2010, 12, 3670-3673. DOI: https://doi.org/10.1021/ol101466g
Melgar, G. Z.; Omori, A. T.; Porto, A. L. M., in: Biocatálise e Biotransformação- fundamentos e aplicações, Vol. 1; Marsaioli, A. J.; Porto, A. L. M., Eds., Schoba, Salto-Brazil, 2010, 250-274.
Zabala, A. O.; Chooi, Y.; Choi, M. S.; Lin, H.; Tang, Y. Chem. Biol. 2014, 9, 1576-1586 DOI: https://dx.doi.org/10.1021/cb500284t DOI: https://doi.org/10.1021/cb500284t
Navarro, D. M. A. F.; Navarro, M. Quim. Nova. 2004, 27, 301-307. DOI: https://doi.org/10.1590/S0100-40422004000200021
Ferreira, I. M.; Rocha, L. C.; Yoshioka, S. A.; Nitschke, M.; Jeller, A. H.; Pizzuti, L.; Seleghim, M. H. R; Porto, A. L. M. Biocatal. Agric. Biotechnol. 2014, 3, 358–364. DOI: https://doi.org/10.1016/j.bcab.2014.04.001
?yszka, B.; Aniol, M.; Lipok, J. Microb. Cell. Fact. 2017, 16,136. DOI 10.1186/s12934-017-0752-3 DOI: https://doi.org/10.1186/s12934-017-0752-3
Takahashi, J. A.; Gomes, D. C.; Lyra, F. H.; Santos, G. F.; Martins, L. R. Molecules. 2014, 19, 1856-1886 DIO: https://doi.org/10.3390/molecules19021856
Pilli, R. A. Quim. Nova Esc. 2001, 14, 16-24.

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









