Journal of the Mexican Chemical Society, vol. 65, no. 2, 2021
Sociedad Química de México A.C.
Maysoon H. Zaboon
University of Basrah, Iraq
Afrodet A. Saleh
University of Basrah, Iraq
Hadi S. Al-Lami *
University of Basrah, Iraq
Received: 15 July 2020
Accepted: 16 December 2020
Abstract: The presence of reactive primary amines in the backbone structure of chitosan enables the derivatization with different functional groups and thereby improving and expanding its properties, such as solubility and mucoadhesiveness, for biomedical applications. In this work, chitosan was grafted with different sources of amino acids (Histidine, Aspartic acid, Glutamic acid, Glycine-Aspartic acid, and Glycine-Glutamic acid), Chitosan and its grafted amino acid derivatives were obtained in very good yield, and they were characterized by Fourier-Transform Infrared Spectroscopy (FTIR), and the resulted spectra confirmed the right structures of chitosan and its different synthesized derivatives. The chitosan and its amino acid derivatives were converted to nanoparticles in size by subjecting them to the sonication method. The Scanning Electron Microscope (SEM) was used to determine the shape and size of the prepared polymeric nanoparticles and the average nanoparticle size counted by the Image-J program. The micrographs revealed that the nanoparticles with spherical shapes and with different sizes were gained, but in general, they are less than 100nm in diameters. In vitro cytotoxicity of chitosan and chitosan derivatives prepared NPs were determined as MTT assay, against different three types of human breast cancer cell lines which are BT cell lines, MCF-7 cell lines, and SKBR3 cell lines. The cell proliferation of each type of breast cancer cell line has appeared to a highly significant decrease (p<0.001), with all types of tested NPs polymers in comparison with the positive control samples, through different periods of the experiment (24, 48, and 72 hours).
Keywords: Chitosan-grafted-amino acids, nanoparticles, breast cancer cell lines, cytotoxicity, MTT.
Resumen: La presencia de aminas primarias reactivas en la estructura del quitosano permite su funcionalización con diferentes grupos funcionales, mejorando y expandiendo sus propiedades, por ejemplo, solubilidad y mucoadhesividad, para aplicaciones biomédicas. En este trabajo se injertó quitosano con diferentes fuentes de aminoácidos (histidina, ácido aspártico, ácido glutámico, glicina-ácido aspártico y glicina-ácido glutámico). Los derivados de quitosano injertados con aminoácidos se obtuvieron con muy buen rendimiento. La caracterización por espectroscopía infrarroja (FTIR) confirmó la funcionalización del quitosano. Después de sonicación y una caracterización por microscopía electrónoica de barrido (SEM), se confirmó que el tamaño del quitosano y sus derivados con aminoácidos pueden clasificarse como nanopartículas. Las micrografías revelaron que las nanopartículas tienen formas esféricas y son de diferentes tamaños, pero en general, son menores a 100 nm de diámetro. La citotoxicidad in vitro de las nanopartículas de quitosano y derivados de quitosano se determinó como ensayos MTT frente a tres tipos diferentes de líneas celulares de cáncer de mama humano, a saber, líneas celulares BT, líneas celulares MCF-7 y líneas celulares SKBR3. La proliferación celular de cada tipo de línea celular de cáncer de mama mostró una disminución significativa (p <0.001), con todos los tipos de polímeros NP probados en comparación con las muestras de control positivo, a lo largo de diferentes períodos del experimento (24, 48, y 72 horas).
Palabras clave: Quitosano funcionalizado con aminoacidos, nanoparticulas, líneas celulares de cáncer de mama, citotoxicidad, ensayos MTT.
Introduction
Chitosan (CS) is a natural cationic polymer. Chitosan is a linear polysaccharide deacetylates from chitin. The abundance, availability, and low cost of chitosan promote its use in a wide variety of applications [1,2,3]. There are two types of reactive functional groups present in chitosan (amino group and a hydroxyl group) that can be used chemically to alter its properties under mild conditions to synthesis different derivatives [4]. Such derivatives can be exploited with good results in several biomedical areas, including enhancement of nucleic acid transfection in gene therapy, as well as many other applications aiming to maximize drug delivery and aiding tissue engineering. The degree of deacetylation is a major factor determining the characteristics of chitosan [5,6]. The natural, biodegradable chitosan polymer can easily undergo an effective complexation synthesis process with DNA to form a stable chitosan-DNA complex [7,8].
A variety of modifications have been investigated to improve chitosan’s properties increasing biocompatibility. It shows good properties as gene delivery vectors and for other medical applications, and one technique used is grafting reactions with some other materials, like anhydride, and poly (ethylene glycol), etc. [3]. Cationic polymers have been improved to increase the efficiency of DNA transfer. The systems will provide an effective tool for gene therapy in the future [9].
Polymeric nanoparticle systems appeared very useful in biomedical applications, for many important scientific reasons. They are easy to prepare from well-understood polymers and have high stability in biological fluids as well as during storage, and the most used in the field of tumors as an anticancer treatment [10]. The polymers utilized to produce these Nanocarriers may be either of natural or of synthetic origin. These polymeric NPs are capable of an extensive variety of medications for a continued timeframe in a controlled manner at target sites to provide enhanced antitumor effectiveness with minimal systemic side effects [11,12].
Various polymers have been used to enhance cellular uptake of NPs, including poly-l-lysine (PLL), which also applies to gene delivery and cell labeling. The addition of the PLL to cultures enhances NPs internalization by stem cells and tumor cells; it is also used in the in vitro and in vivo therapy of cancer. This biodegradable polymer also forms Nano size complexes (< 100 nm) with polynucleotides owing to the presence of protonated amino groups on the lysine moiety. Polyplexes of the PLL are reported to have good cellular uptake [13,14].
In recent years, chitosan has received increased attention for its commercial applications in the biomedical, chemical, and food industries. In studies on the inherent properties of chitosan, it appears, it has several inherent properties such as bactericidal, fungicidal activity, and wound healing potential. Chitosan possesses many interesting characteristics due to its availability and highly flexible chemical compositions and properties ease of extraction, chemical modifications, and low cost, therefore, it is widely used in biomedical applications [15]. Chitosan and chitosan derivatives display good anticancer effects with a decrease in the adverse effects of the original drug due to a predominant distribution into cancer and a gradual release of free drug from the conjugates [12]. Thus, they are successful in cancer therapy and reducing the damaging nonspecific side effects of chemotherapeutic. Besides, the formulation of these nanoparticles with imaging contrast agents provides a very efficient system for cancer diagnostics [16].
Recent advances in nanotechnology promise further developments in target-specific drug delivery systems [17], therefore, the ongoing work concentrated on developing cancer-specific drugs or delivery systems in form of nanoparticles can preferentially localize existing agents to the tumor site. This involved interacting chitosan with different sources of amino acids (i.e. Histidine, Aspartic acid, Glutamic acid, Glycine-Aspartic acid, and Glycine-Glutamic acid) to study their cytotoxicity in term of MTT assay in three different human breast cancer cell lines (BT, MCF7, and SKBR3) as antitumor agents. Various methods can be used to indirectly measure the quantity survival and proliferation rate of cells. Most of these methods measure the activity rate of a cellular enzyme inside a living cell. MTT assay is one of the most recognizable methods to do so.
Experimental
Materials and measurements
Chitosan was obtained from the extracted chitin of the local shrimp cortex with a high degree of deacetylation (DD = 92.1) [3]. Amino acids with 99% purity were purchased from different companies, Histidine and Glutamic acid (Merck Company), Aspartic acid and Glycine (BHD Company), all they were used in the grafting of chitosan without further treatment. Dimethyl sulfoxide (DMSO), methanol, dioxane, acetic acid, Glutaraldehyde were purchased from Sigma-Aldrich Company.
Fourier-transform infrared (FTIR) measurements in the range of 500-4,000 cm-1 were obtained by using a potassium bromide disc on Shimadzu model FTIR-8101M spectrophotometer (Japan). The size and morphology of chitosan and its derivative nanoparticles were identified using ZEISS (Germany) scanning electron microscopy (SEM).
Methods
Preparation of chitosan-grafted-histidihn (Cs-g-His)
CS-g-His was prepared by reaction between 1g of chitosan with 2g histidine dissolved in 50 mL dry DMSO, and then they heated to145 °C for 24 hours in a dry nitrogen environment. The chemical reaction is shown in Scheme 1. The hot mixture was left to cool down to ambient temperature, and the solid product was filtered and washed with methanol several times. It was dried in the vacuum desiccator [18]. The white powder product of (CS-g-His) was obtained with an 80.7% yield.
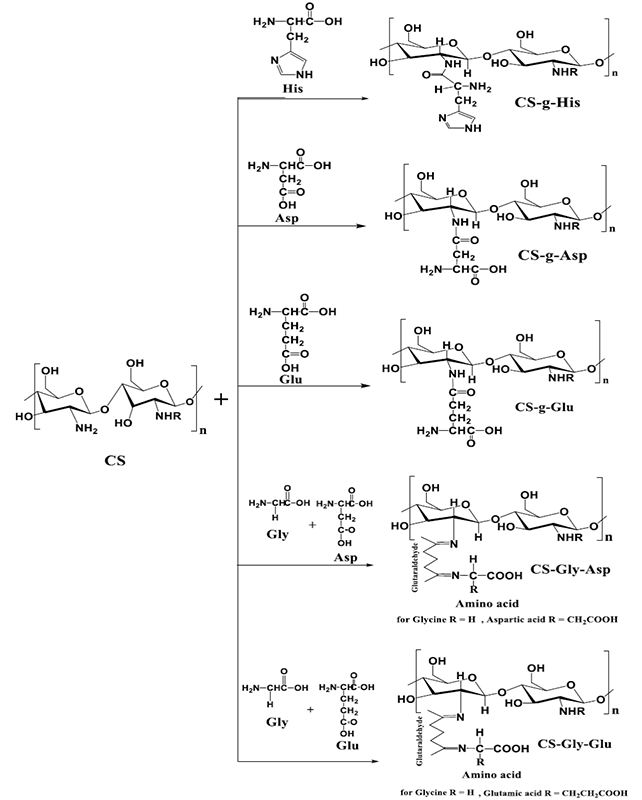
Scheme 1
Chemical grafting reactions of Chitosan with different amino acids.
Preparation of chitosan-grafted-aspartic acid (CS-g-Asp)
CS-g-Asp was prepared by reaction between 1g of chitosan with 3g aspartic acid dissolved in 50 mL dioxane; the pH of the suspension was controlled to 9.5-10 using 1M NaOH solution, and then it was refluxed at 101 °C for 18 hours. Finally, the product was cooled to room temperature, Scheme 1. The white powder was filtered and washed with methanol many times, then dried in the vacuum desiccator [19]. (CS-g-Asp) was obtained with an 87 % yield.
Preparation of chitosan-grafted-glutamic acid (CS-g-Glu)
CS-g-Glu was prepared by the reaction between 1g of chitosan with 3g glutamic acid dissolved in 50 ml dioxane, the pH of the suspension was controlled to 9.5-10 using 1 M NaOH solution, and the mixture was refluxed heated up at 101 °C for 18 hours, Scheme 1. Finally, the product was cooled down to room temperature, the product filtered and washed with methanol several times. It was dried in the vacuum desiccator [19]. The white powder product of (CS-g- Glu) was obtained with 85 % yield.
Preparation of chitosan-glycine-aspartic acid (CS-Gly-Asp)
CS-Gly-Asp was prepared by the reaction between 1g chitosan and a mixture of two amino acids (0.5 g Glycine and 0.5 g Aspartic acid) dissolved in 50 mL of 1 % acetic acid by weight and magnetically stirred for 3h at room temperature. The homogeneous mixture was extruded in the form of droplets using a syringe into NaOH-methanol solution (1:20 w/w) under a stirring condition at 400 rpm. The resultant beads were allowed to react with (6.25 %) glutaraldehyde solution at 50 °C for about 10 minutes after washing with water, Scheme 1 represents the chemical reaction. Finally, the beads were washed with hot and cold water, followed by drying in the vacuum desiccator [20,21]. The yellow powder product of (CS-Gly-Asp) was obtained with a 77 % yield.
Preparation of chitosan-glycine-glutamic acid (CS-Gly-Glu)
CS-Gly-Glu was prepared by the reaction between 1g chitosan, and a mixture of amino acids (0.5 g Glycine and 0.5 g Glutamic acid) dissolved in 50 mL of 1 % acetic acid by weight and magnetically stirred for 3hours at room temperature, Scheme 1. The homogeneous mixture was extruded in the form of droplets using a syringe into NaOH-methanol solution (1:20 w/w) under a stirring condition at 400 rpm. The resultant beads were allowed to react with (6.25 %) glutaraldehyde solution at 50 °C for about 10 minutes. Finally, they washed with hot and cold water, followed by drying in the vacuum desiccator [20,21]. The yellow powder product of (CS-Gly-Glu) was obtained with a 76.3 % yield.
Preparation of chitosan and chitosan derivatives nanoparticles
Chitosan and chitosan-amino acid derivatives were dissolved with 2 % acetic acid solution pH = 3. Then, the solution was subjected to sonication technique for 5 min at 50 W with a pulse of 5 sec and 10 sec between pulses [22]. The produced nanoparticles were characterized by Scanning Electron Microscope, SEM, (ZEISS, Germany) adjusted at 100 kV. Image-J program was used to estimate the mean size and total account of each polymer NPs.
The cytotoxicity assay
The cytotoxicity assay was evaluated in three different types of human breast carcinoma cell lines which are: MCF-7, BT, and SKBR-3, protected in an appropriate vehicle for each sort, at 37 °C in a moist 5 % CO2 environment. The cells were cultured in a 96-well plate (7500 cells/well) and permitted to grow for 24 hours. Then 1mg/mL of each polymer nanoparticle solution was applied to the already designated wells and further incubation was carried out in 24, 48, and 72 hours, respectively. Afterward, the viability of the tested cells was estimated by using MTT. Absorbance at 550 nm was measured with the Tecan plate reader, and reference at 620 nm. The MTT value of untreated cells positive control (human breast cancer cell lines type MCF-7, BT, and SKBR-3without polymer) was considered as 100 % cell viability. All transfection and cytotoxicity assays were performed in triplicate [23].
The quantitative results data obtained from the cytotoxicity tests were analyzed using the Student t-test for comparative mean values; very significant differences at the level of p < 0.001 were acquired.
Statistical analysis
All samples were evaluated in triplicates and were presented as (means ± SD). For statistical analysis, the variance (ANOVA) test was used to assess the significance between the groups. Differences were regarded as statistically highly significant if P < 0.001.
Results and discussion
Synthesis of amino acids-grafted-chitosan
The grafting of different amino acids onto chitosan was carried out successfully to enhance the bioactivity and broaden the applications of chitosan. The main objective of the present work is to study the cytotoxicity of the prepared grafted chitosan by MTT assay. The structural characterization of all amino acid-g-chitosan is the key to use these cationic polymers. They were characterized by FTIR and their spectra were recorded by Shimadzu 8400 FTIR/Japan.
FTIR characterization of the prepared chitosan-grafted amino acids
The FT-IR spectrum of the CS-g-His shows the index peaks of 3429-3383 cm-1 assigned for (-O-H) and (-N-H) stretching vibrations, 2924-2854 cm-1 (-C-H) stretching vibrations, 1656 cm-1 (-N-H) bending vibrations, 1419 cm-1 (-C-N) stretching vibrations and 1029 cm-1 C-O-C stretches vibrations are assignable to chitosan. New absorption peaks for histidine appeared at 3078 cm-1 (-C-H) stretching vibrations, 1797 cm-1 and 1616 cm-1 for (-C=O) and (-C=N) stretching vibrations respectively, which confirm the synthesis of CS-g-His [13]. CS-g-Asp FTIR spectrum illustrates an absorption band at 1747 cm-1 which is characteristic for the amide linkage -CONH, which proves the reaction between one of the carboxylic groups of glutamic acid and NH2 of chitosan by a condensation reaction. On the other hand, the absorption bands at 1700-1600 cm-1 assigned for N-H bending, 1562 cm-1 C-N stretching, and 2951 cm-1 aliphatic C-H. The band that appears at 1438 cm-1 can confirm that the other carboxylic group of glutamic acid is still free and not reacted. The CO is stretching band of acid was present at 1222 cm-1 and 1562 cm-1 for an N-H stretch band of the amino group [18].
The same was true for the peaks appeared in the CS-g-Glu FTIR spectrum, and they are as follows; band at 1739 cm-1 characteristic for the amide linkage -CONH, bands at 1700-1600 cm-1, 1593 cm-1, and 2885 cm-1 are assigned for N-H bending, C-N stretching and aliphatic C-H respectively. The peak that appears at 1419 cm-1 can confirm that the other carboxylic group of glutamic acid is still free and not reacted. Besides, C-O the stretching band of acid was present at 1280 cm-1 and 1593 cm-1 for an N-H stretch band of the amino group [24].
Grafting Chitosan with a mixture of Glycine and Aspartic acid (CS-Gly-Asp) or with Glycine amino acid Glutamic amino acid (CS-Gly-Glu) revealed a presence of a new peak, in addition to the above-discussed peaks, at about 1564 cm-1 due to the imine bond (-C=N-) which was formed as a result of cross-linking reaction between the amino group in chitosan and aldehyde group in glutaraldehyde. This was due to the overlapping of peaks corresponding to (-NH) stretching vibrations in (-NH-COCH3) of the original chitosan with that of imino (-C=N-) stretching at 1564 cm-1 of the newly formed structure between the amino group of chitosan and an aldehyde group of glutaraldehyde. A reaction takes place in the formation of crosslinks as follows [20]:
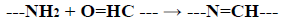
Scanning electron microscopy of chitosan and its derivatives nanoparticles
SEM micrographs and ImageJ results verified the formation of Nanosize and spherical shape of pure chitosan nanoparticles and their derivatives. Fig. 1(A) shows the SEM micrograph of the purified CS as small spherical nanoparticles with a minimum and a maximum size ranging from 15-82 nm as counted by the Image-J program. This program is used for the analysis of the image obtained from TEM or SEM, which is the case here. It correlates the number of particles counted and their minimum and maximum size by giving the mean particle size of the nanoparticles prepared, as shown in Fig. 1(B); while the SEM image of the representative CS-g-His, Fig. 2(A) also showed a spherical shape, that their counted particle size ranging from 24-85 nm, Fig. 2(B). The same was true for the other representative chitosan grafted with a combination of Glycine and Glutamic acid, Fig. 3(A), and the ranging size calculated the Image-J program appeared between 24-93 nm, Fig. 3(B).
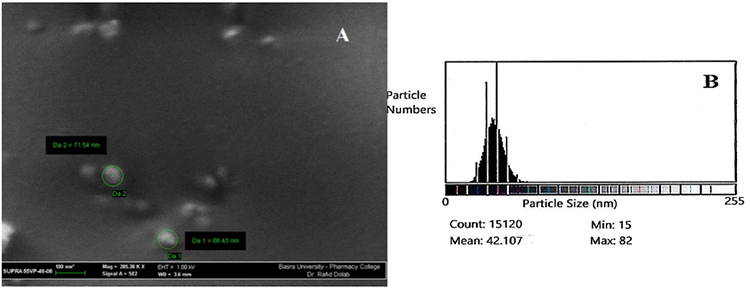
Fig. 1
(A) SEM micrograph of prepared chitosan nanoparticles, (B) Size of Chitosan nanoparticles using Image-J program.
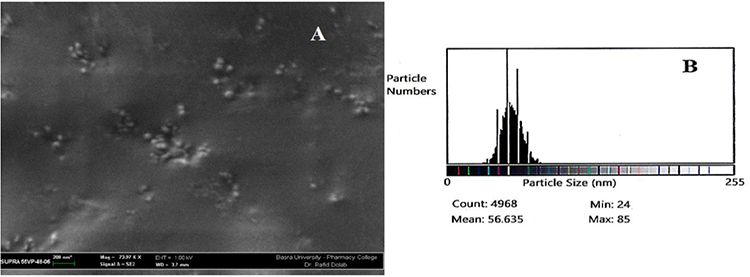
Fig. 2
(A) SEM micrograph for the grafted CS-g-His derivative, (B) Size of the CS-g-His derivative nanoparticles using Image-J program.
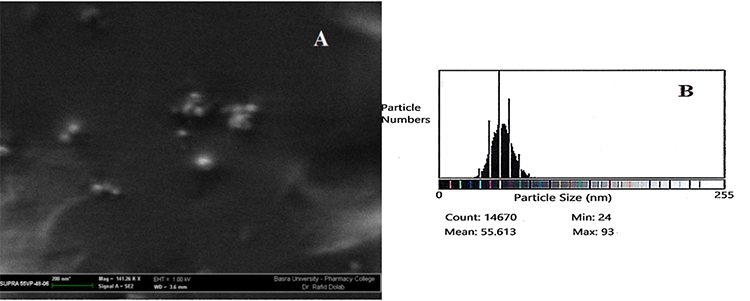
Fig. 3
(A) SEM micrograph for the grafted CS-g-Gly-Glu derivative, (B) Size of the CS-g-Gly-Glu derivative nanoparticles using Image-J program.
MTT assay
It is worth mentioning here, all the chitosan grafted amino acids were converted to nanoparticles in size by subjecting them to the sonication method [22,25], and the scanning electron microscope (SEM) was used to determine the shape and size of the prepared polymeric nanoparticles. The micrographs revealed that the nanoparticles with spherical shapes, as shown in Fig. 1. The surface of the sample showed bead-like structures indicating a change has occurred. This is indicative of the use of the proper method in the preparation of polymeric Nanoparticles.
MTT assay is a quantitative test used to measure the proliferation rate of cells in the face of different factors and to determine toxicity levels of these factors in the cells. This assay depends on the ability of the mitochondrial reductase enzyme of living cells to resuscitate and transform MTT (3-(4, 5-Dimethylthiazol 2-yl)-2,5- diphenyltetrazolium bromide) yellow tetrazolium rings with insoluble purple crystals of formazan [26].
The stimulation of in vitro cytotoxicity effect of the CS-His, CS-Asp, CS-Glu, CS-Gly-Clu, and CS-Gly-Glu nanoparticles carried out in different human breast cancer cell lines, as a cell viability assay. The comparative results of these amino acid derivatives indicating a presence of different cytotoxicity patterns of these derivatives; but still highly significant, p<0.001, in decreasing cell growth in comparison with control groups. The growth manner of the cell viability of all types of breast carcinoma as a percentage of cell viability after treatment with various chitosan-amino acid derivatives prepared in this work are represented in Table 1 and Figures 4 and 5.
The mean population triplicate time (PTT) ± standard deviation (SD) values as the antitumor effect of prepared polymer nanoparticles against the proliferation of human breast cancer cell lines BT cells, MCF-7 cells, and SKBR3, with P < 0.001.
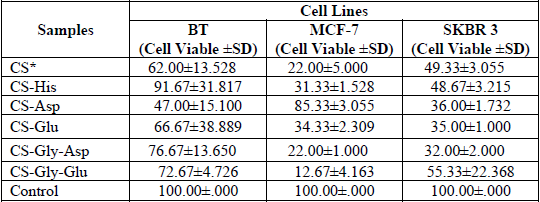
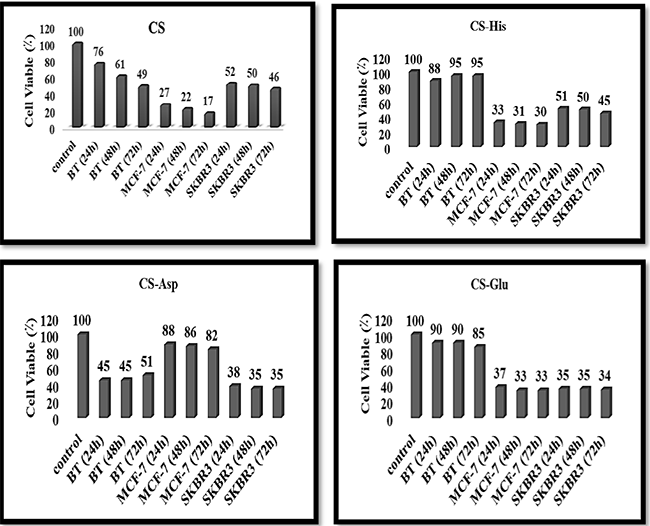
Fig. 4
Cell viability percentages of human breast cancer cell lines (BT cells, MCF-7 cells, and SKBR3 cells) affected with CS [29], CS-His, CS-Asp, and CS-Glu NPs at different times for 24h, 48h, and 72h.
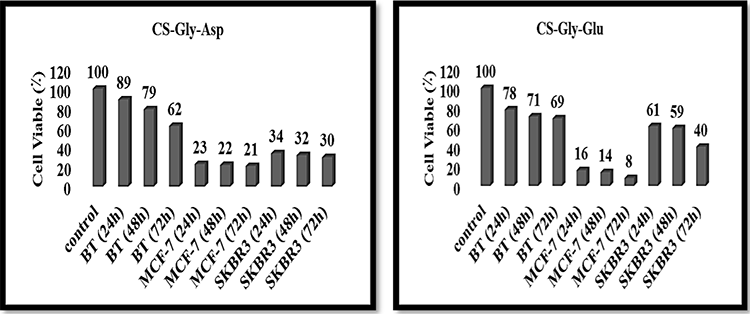
Fig. 5
Cell viability percentages of human breast cancer cell lines (BT cells, MCF-7 cells, and SKBR3 cells) affected with CS-Gly-Asp and CS-Gly-Glu NPs at different times for 24h, 48h, and 72h.
This investigation revealed that chitosan-amino acid derivatives nanoparticles are significant against the growth progression of human breast cancer MCF-7, BT, and SKBR-3 cell lines. These investigations agreed well with Casettari et al. [27]. They reported that the inclusion of amino acid molecules to the backbone of CS has been improving the physicochemical properties of CS such as solubility and gives rise to some interesting synergistic characteristics for use in drug delivery and tissue engineering, as well as other potentially useful biomedical properties such as anticancer, anticoagulant, antimicrobial, and cholesterol-lowering activities. Besides, it was reported that the amino acid with a cationic surface charge promoted the internalization ability of amino acids-grafted CS NPs [28], through the electrostatic interaction between positively surface-charged NPs and negatively charged cell membrane [29,30]. Hence, these results agreed very well with that described by Layek and Singh [31], and they showed that the cellular uptake and transfection efficiency improved with the increased amino acid hydrophobicity.
Conclusion
Our results showed that true grafting processes successfully carried out to synthesize obtained different derivatives of chitosan with some amino acids. Self-assembled NPs based on CS-g-His, CS-g-Asp, CS-g-Glu, CS-g-Gly-Asp, and CS-g-Gly-Glu conjugate developed by sonication method, and they showed narrow size distributions as examined by SEM. The cytotoxicity results represented just a promising start for use of biocompatible amino acids grafting chitosan nanoparticles to improve their features for numerous medical applications, especially for human breast cancer therapy. The grafted CS-amino acids derivatives enhanced cell uptake by transfection method which used in the MTT assay that used with human cell lines to transfer and uptake the nanoparticles, with a different growth inhibition against the three various types of human breast cancer cell lines tested; BT cell lines, MCF-7 cell lines and SKBR3 cell lines. The cell proliferation of each breast cancer cell lines type appeared highly significant decreasing (p<0.001), with all types of tested NPs polymers in comparison with the positive control samples which represented all three types of human breast cancer cell lines (MCF-7, BT, and SKBR-3) untreated with nanoparticles, and they also improved bioavailability over native CS. This study also demonstrated the impact of chitosan-g-amino acid derivatives nanoparticles as cationic biocompatible polymers are exerting in vitro less cytotoxicity effect on the studied human breast cancer cell lines and did not show any or less effect on human DNA within 1 mg/ml of each concentration. However, decreasing the concentration of these NPs polymers can be promising candidates as cancer gene therapy and therapeutic agents as well.
References
1. Song, Z.; Li G.; Guan, F.; Liu, W. Polymers 2018, 10, 389-402. DOI: https://doi.org/10.3390/polym10040389
2. Ibitoye, E. B.; Lokman, I. H.; Hezmee, M. N. M.; Goh, Y. M.; Zuki, A. B. Z.; Jimoh, A. A. Biomed. Mater. 2018, 13, 1-13. DOI: https://iopscience.iop.org/article/10.1088/1748-605X/aa9dde
3. Mutasher, S. H.; Salih, A. A.; Al-Lami, H. S. Der. Pharma. Chemica 2016, 8, 125-134. http://derpharmachemica.com/archive.html
4. Li, Z.; Yang, F.; Yang, R. Int. J. Biol. Macromol. 2015, 75, 378-387. DOI: https://doi.org/10.1016/j.ijbiomac.2015.01.056
5. Guţoaia, A. Chitosan Nanoplexes for target siRNA deliver, Ph.D. thesis, the University of Tübingen, aus Galaţi, Rumänien, 2015. https://publikationen.uni-tuebingen.de
6. Raftery, R; O’Brien, R. F. J.; Cryan, S. Molecules 2013, 18, 5611- 5647. DOI: https://pubmed/10.3390/molecules18055611
7. Jennings, J. A.; Bumgardner, J. D. Chitosan Based Biomaterials, Volume 2: Tissue Engineering and Therapeutics, 1st ed.; Elsevier Ltd.: United Kingdom, 2017, 1-10. https://www.elsevier.com/books/chitosan-based-biomaterials-volume-2/jennings/978-0-08-100228-5
8. Carballal, B.; Fernández, E. F.; Goycoolea, F. M. Polymers (Basel) 2018, 10, 444, 1-51. DOI: https://ncbi/10.3390/polym10040444
9. Zhang, P.; Wagner, E., Top Curr. Chem. (Z) 2017, 375, 1-39. DOI: https://pubmed.ncbi/10.1007/s41061-017-0112-0
10. Gad, A.; Kydd, J.; Piel, B.; Rai1, P. Int. J. Nanomed. Nanosurg. 2016, 2, 1-12. DOI: https://ncbi/10.16966/2470-3206.116
11. Prabhu, R. H.; Patravale, V. B.; Joshi, M. D. Int. J. Nanomed. 2015, 10, 1001-1018. DOI: https://ncbi/10.2147/IJN.S56932
12. Chen, G.; Gong, R. Saudi Pharm. J. 2016, 24, 250-253. DOI: https://ncbi/10.1016/j.jsps.2016.04.008
13. Lounis, F.M.; Chamieh, J.; Leclercq, L.; Gonzalez, P.; Rossi, J.; Cottet, H. Polymers 2018, 10, 45, 1-16. DOI: https://mdpi/10.3390/polym10010045
14. Hong, C. A.; Sonm, H. Y.; Nam, Y.S. Nature Sci. Rep. 2018, 8, 7738, 1-7. DOI: https://nature/10.1038/s41598-018-25655-7
15. Perni, S.; Prokopovich, P. Nanom.: Nanotech. Bio. Med. 2017, 13, 539-548. DOI: https://doi.org/10.1016/j.nano.2016.10.001
16. Gabano, E.; Quental, L.; Perin, E.; Silva, F.; Raposinho, P.; Paulo, A.; Ravera, M. Inorganics 2018, 6, 1-13. DOI: https://doi.org/10.3390/inorganics6010004
17. Alshraim, M. O.; Sangi, S.; Harisa, G. I.; Alomrani, A. H.; Yusuf O.; Badran, M. M. Molecules 2019, 24, 1-14.DOI: https://pubmed.ncbi/10.3390/molecules24020250
18. Hajipour, A. R.; Hosseinia, S. M.; Jajarmi, S., New J. Chem. 2017, 41, 7447-7452. DOI: https://doi.org/10.1039/C7NJ00595D
19. Galhoum, A. A.; Hassanc, K. M.; Desouky, O. A.; Masoud, A.; Akashid, M. T.; Sakaid Y.; Guibalb, E. React. Funct. Polym. 2017, 113, 13-22. DOI: https://doi.org/10.1016/j.reactfunctpolym.2017.02.001
20. Rani, M.; Agarwal, A.; Negi, Y. S. J. Biomat. Nanobiotech. 2011, 2, 71-84. DOI: http://SciRP.org/10.4236/jbnb.2011.21010
21. Rani, M.; Agarwal, A.; Maharana, T; Negi, Y. S. Afr. J. Pharm. Pharmacol. 2010, 4, 35-54. URL: https://academicjournals.org/journal/AJPP/article-full-text-pdf/A86C79232822
22. Silva, V. J. D. Preparation and characterization of chitosan nanoparticles for gene delivery, M.Sc. thesis, University of Lisbon, Portugal, November 2013, 31-32. https://fenix.tecnico.ulisbon.pt
23. Mosmann, T. J Immunol Methods 1983, 65, 55-63. DOI: https://doi.org/10.1016/0022-1759(83)90303-4
24. El-Ghaffar, M. A. A.; Akl, M. A.; Kamel, A .M.; Hashem, M. S., Egypt J. Chem. 2017, 60, 507-518. DOI: https://ejchem.journals.ekb.eg/10.21608/ejchem.2017.745.1021
25. Zaboon, M. H. Synthesis and Characterization of some New Polymeric Chitosan Nanoparticles Derivatives as Antitumor for Human Breast Cancer Cell Lines, Ph.D. thesis, University of Basrah, Iraq , Janaury 2019, 44-63.
26. Ferrari, M.; Fornasiero, M. C.; Isetta, A. M. J. Immun. Meth. 1990, 131, 165-172. DOI: https://pubmed.ncbi.10.1016/0022-1759(90)90187-z
27. Casettari, L.; Vllasaliu, D.; Lam, J. K.; Soliman, M; Illum, L. Biomaterials 2012, 33, 7565-7583. DOI: https://doi.org/10.1016/j.biomaterials.2012.06.104
28. Al-Lami, H. S.; Zaboon, M. H.; Saleh, A. A. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 871, 1-15. DOI: https://iopscience.iop.org/article/10.1088/1757-899X/871/1/012024
29. Wernersson, E.; Heyda, J.; Kubícková, A.; Krízek, T.; Coufal, P.; Jungwirth, P. J. Phys. Chem. B. 2010, 114:11934-11941. DOI: https://doi.org/10.1021/jp1054342
30. Raja, M. A.; Zeenat, S. Arif, M.; Liu, C. Int. J. Nanomed. 2016, 11, 4397-4412.DOI https://doi.org/10.2147/IJN.S106116
31. Layek, B.; Singh, J. Biomacromolecules 2013, 14, 485-494. DOI: https://doi.org/10.1021/bm301720g
Author notes
*Corresponding author: Hadi Salman Al-Lami, email: hadisalman54@yahoo.com, mobile: +9647901681631

 cygnusmind
cygnusmind