Journal of the Mexican Chemical Society, vol. 65, no. 3, 2021
Sociedad Química de México A.C.
Ayman. M. Algohary
Department of Chemistry, College of Science Al-zulfi, Majmaah University, P.O. 66 Al-Majmaah, 11952, Saudi Arabia., Saudi Arabia
Egyption Drug Authority (EDA), P.O.29 Giza, Egypt., Egypt
Mohamed M. Hassan *
Ain Shams University, Egypt
Sami G. Almalki
Medical Laboratory Department, College of Applied Medical Sciences, Majmaah University, P.O. 66 Majmaah11952, Saudi Arabia., Saudi Arabia
Esam S. Al-Malki
Department of Biology, College of Science Al-zulfi, Majmaah University, P.O. 66 Al-Majmaah, 11952, Saudi Arabia., Saudi Arabia
Received: 14 January 2021
Accepted: 30 March 2021
Abstract: The current project deals with designing and synthesizing of colorimetric chemosensors to detect the cations in the aqueous medium and biological sample. To achieve this goal a new series of quinazolinone derivatives were synthesized via reaction of the novel 6-nitro-2-propyl-4H- benzo[d][1,3]oxazin-4-one (3) with selected nitrogen nucleophiles, namely, formamide, hydrazine hydrate, hydroxylamine hydrochloride, O-phenylendiamine, O-aminophenol and O-aminothiophenol, urea and/or thiourea. Structures of the new compounds have been investigated depending on their spectral data (IR, 1H NMR, 13C NMR and MS) and elemental analyses. Some of the newly synthesized products exhibited a significant response as chemosensors for some cations detection. The synthesized chemosensors 11a and 11b showed high-selectivity and specificity towards cooper (CuII) and mercury (HgII) cations detection through exhibiting colormetric responses. Chemosensors 7 and 10 b showed high selectivity toward cadmium (CdII) cation, whilst other examined compounds (9b, c, 10a, 12, 13, and 14) did not exhibit colorimetric response in all cation's samples.
Keywords: Chemosensor, quinazolinone, copper, cadmium, mercury.
Resumen: En el presente proyecto se diseñan y sintetizan quimiosensores colorimétricos para detectar los cationes en el medio acuoso y en la muestra biológica. Para lograr este objetivo se sintetizó una nueva serie de derivados de quinazolinona mediante la reacción de la 6-nitro-2-propil-4H-benzo[d][1,3]oxazin-4-ona (3) con nucleófilos nitrogenados seleccionados, a saber, formamida, hidrato de hidracina, clorhidrato de hidroxilamina, O- fenilendiamina, O -aminofenol y O-aminotiofenol, urea y/o tiourea. Las estructuras de los nuevos compuestos se han comprobado en función de sus datos espectrales (IR, 1H NMR, 13C NMR y MS) y de los análisis elementales. Algunos de los nuevos productos sintetizados mostraron una respuesta significativa como quimiosensores para la detección de algunos cationes. Los quimiosensores sintetizados 11a y 11b mostraron una alta selectividad y especificidad hacia la detección de los cationes cobre (Cu II) y mercurio (Hg II) al mostrar respuestas colormétricas. Los quimiosensores 7 y 10b mostraron una alta selectividad hacia el catión cadmio (Cd II), mientras que otros compuestos examinados (9b, c, 10a, 12, 13 y 14) no mostraron respuesta colorimétrica con los cationes investigados.
Palabras clave: Químiosensor, quinazolina, cobre, cadmio, mercurio.
Introduction
Currently, synthesis of colorimetric sensors for detecting of cations and anions is one of the researcher's targets in the organic synthesis field because of the prospect implementation of these chemosensors as diagnostic tools in medical, physiological and environmental applications [1]. Cooper cation (CuII), is the most abundant ion in the human body and several proteins utilize CuII as a cofactor for the transfer of electrons in redox reactions. Biologically, excess Cu II in cells can stimulate the creation of reactive oxygen species, and can harm lipids, DNA and RNA and cause some of the dangerous diseases, such as Dementia's disorder, Prion, Wilson and Menkes disease, which are directly related to the copper toxicity [2]. Also, cadmium cation (CdII) is extremely poisonous to the living organism and its compounds pass in the environment from human activities or geological. Cadmium and its salts are listed as blacklist compounds, since elevated levels of CdII exposure are associated with an increase in cardiovascular risk, cancer, liver disease and kidney disorder [3]. On the other hand, mercury cation (HgII) is considered as the greatest harmful pollutant, which affects directly on human and environmental hea lth, so assay of mercury ions in the environment is a significant target reference [4]. The most reports of sensing (HgII) based on using atomic absorption spectrometry, raman spectroscopy, and inductively coupled plasma mass spectrometry, which are expensive tools and needs long time pre- treatment [5]. Various techniques such as electrochemical, atomic absorption, inductively coupled plasma atomic emission and piezoelectric quartz crystals [6], have been used for notifying of metal ions but their utilization is limited due to its expensive equipment, laboratories and time consuming procedures. Weiying et al. have applied a ratiometric fluorescent probe for CuII determination [7]. Takunori et al., used isotope dilution inductively coupled plasma mass spectrometry for the detection of silver copper, zinc, nickel, lead, and cadmium in seawater [8].
Recently, colorimetric techniques are the most favorable methods due to their low cost, lack of equipment, rapid, in addition to its naked eye noteworthy as the production of colorimetric chemosensors depends on that the change in absorption accompanied by appropriate changes in colour [9]. Ha et al., showed colorimetric sensor for the determination of CdII, HgII, and PbII ions [10]. Meng et al., utilized a turn-on fluorescent sensor for assay of CdII, HgII, and ZnII in water [11]. Umesh et al., showed selectivity of dual channel chemosensor for copper in semi aqueous media [12]. In the last decade, numerous heterocyclic chemosensors for the identification of different ions have been synthesised. Xue-Jiao et al., synthesised heterocyclic chemosensors based on azo dye to generate fluorescent sensors for zinc detection without cadmium interference [13]. Chunliang et al., designed a sensor containing naphthalimide as the fluorophore, which by undergoing two reverse intraligand charge transfer (ICT) processes in sensing ZnII and CdII distinguished between these ions pair proficiently [14].
Quinazolinones have attracted notable interest in organic and medicinal chemistry due to their therapeutic potential such as anticancer, antibacterial, antidiabetic, hypnotic, sedative, analgesic, anticonvulsant, antitussive, anti-inflammatory activities [15]. Few reports were performed for checking the sensor activity of quinazolinone derivatives. Ailin et al., synthesized novel quinazolinone compound as a fluorescence sensor for the ferric ion [16]. Sensors based on quinazolinone have been produced for amine vapours and CuII ion [17]. As an extension of our awareness in synthesis of quinazolinone heterocyclic, we report that some quinazolin-4(3H)-ones can be used as a simple and efficient ‘turn-on’ fluorescent chemosensor for a highly selective and sensitive detection of some cations, CuII , HgII and CdII in aqueous medium and biological sample.
Experimental
General
Melting points were measured on an Optimelt automated melting point system and are uncorrected. The IR spectra were recorded on a Perkin−Elmer 1800 Series FTIR spectrometer. Samples were analyzed as thin films on KBr plates. 1HNMR and 13CNMR spectra were recorded at room temperature in base-filtered DMSO-d6 on a Varian spectrometer operating at 400 MHz for proton and 100 MHz for carbon nuclei. Elemental microanalyses were recorded on a Perkin Elmer series II CHNS analyzer 2400. Mass spectra were obtained using GCMS (QP-1000EX) Shimadzu gas chromatography instrument mass spectrometer (70 eV).
2-(Butyramido)-5-nitrobenzoic acid (2)
Butyryl chloride (1.06 g, 10 mmol) was portion-wise added to a stirred solution of 2-amino-5-nitrobenzoic acid (1.8 g, 10 mmol), in dry pyridine (30 mL) during 30 min. The mixture was stirred at room temperature for 2 h then poured into ice-cold water (200 mL), acidified with hydrochloric acid (2N) up to complete precipitation. The crude solid product was filtered off, washed thoroughly with cold water, and crystallized from benzene to give compound 2; (2 g, 80 %); yellow crystals; m.p. 130-135 oC. IR (KBr), ν (cm-1): 3300 (O-H, N-H), 3010 (C-Harom), 2975, 2985 (C-Haliph), 1730 (C=Ocarboxylic), 1640 (C=Oamidic), 1615 (bending N-H). 1H NMR (CDCl3) δ: 1.90 (t, 2H, -CH2-CH2-CH3), 1.50 (m, 2H, - -CH2ME) and 1.20 (t, 3H, - CH3), 7.20 -8.70 (m, 3H, Harom ), 8.95 (bs, 1H exchangeable with D 2O, NH) , 11.80 (bs, 1H exchangeable with D2O, OH); 13C NMR (100 MHZ) δ 143.3 (C-1), 115.15(C-2), 129.7 (C-3), 122.4(C-4), 132.98(C-5), 121.52 (C-6), 168.1 (C-7COOH), 170.2 (C-8NHC=O), 35.1 (C-9), 19.0 (C-10), 13.2 (C-11) ; MS m/z 252(M+,70), 253 (M+1, 80). Anal. Calcd for C11H12N2O5 (252): C, 52.38; H, 4.80; N, 11.11; O, 31.72. Found: C, 52.50; H, 4.95; N, 31.94.
6-Nitro-2-propyl-4H-benzo[d][1,3]oxazin-4-one (3)
The dry solid anthranilide (2.52g, 10 mmol) 2 was treated with freshly distilled acetic anhydride until being pasted and then heated over water path for 2h before lifting to cool. The separated out solid was filtered off and recrystallized from light petroleum ether 40/60 affording the benzoxazinone 3 . ( 2 g, 85%); pale yellow crystals; m.p 185 oC; IR (KBr), ν (cm -1): 3050 (C-Haromatic), 2971, 2929, 2881 (C-Haliphatic),1710(C=O), 1617(C=N), 1159 (C-O -C); 1H NMR (400 MHZ, DMSO-d6) δ 2.10(2H, -CH2- CH2-CH3), 1.60 (m, 2H, --CH2ME) and 1.10 (t, 3H, -CH 3), 7.3- 8.2 (m, 3H, Ar-H) ; 13C NMR (100 MHZ) δ 155.2 (C- 1C=N), 159.1 (C-2C=O), 120 (C-3), 128 (C-4), 127.4 (C-5), 133.5 (C-6), 122 (C-7), 147 (C-8), 25.1 (C-9), 14.6 (C-10), 13.9 (C-11); MS m/z 234 (M+, 20), 235 (M+1, 18); Anal. Calcd. For C11H10N2O4 (234): C, 56.41; H, 4.30; N, 11.96%. Found: C, 56. 50; H, 4.47; N, 11.99%.
6-Nitro-2-propylquinazolin-4(3H)-one (4)
A solution of benzooxazinone derivative 3 (2.34 g, 10 mmol,) in formamide (15 ml) was refluxed for 2 h, left to cool, then poured into ice. The crude solid product was collected by filtration, dried, and recrystallised from ethanol to give 4. (1.75g ,75%); yellow crystals; mp > 300 °C; IR (KBr), ν (cm-1): 3372, 3280 (NH), 1670 (C=O), 1622(C=N); 1H NMR (400 MHZ, DMSO -d6 ) δ 2.30(2H, - CH2-CH2-CH3), 1.80 (m, 2H, - -CH2ME) and 1.22 (t, 3H, -CH3), 7.82-8.90 (m, 3H, Ar-H), 9.40 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHZ) δ 155.2 (C-1), 164 (C-2), 123 (C-3), 130.5 (C-4), 127.4 (C-5), 133.5 (C-6), 122 (C-7), 148.8 (C-8), 25.4 (C-9), 14.6 (C-10), 13.7 (C-11) MS m/z 233(M+, 30), 234 (M+1, 23); Anal. Calcd for C11H11N3O3 (233): C, 56.65; H, 4.75; N, 18.02. Found: C, 56.80; H, 4.90; N, 18.40.
3-Acetyl-6-nitro-2-propylquinazolin-4(3H)-one (5)
A solution of 4 (2.33 g, 10 mmol) in 20 ml acetic anhydride was refluxed for 4 h. The reaction mixture was then allowed to stand at room temperature for 2 h. The separated solid product was washed with wáter (2x100 ml), dried and crystallized from ethanol to afford compound 5. (1.65 g, 60%); pale-yellow crystals; m.p: 135 -7 oC; IR (KBr), ν (cm-1): 1663 (C=O Acetyl),1675(C=OQuinazolinone), 1622(C=N); 1H NMR (400 MH Z, DMSO-d6) δ: 2.40 (s, 3H, CH3-C=O), 2.00(2H, -CH2-CH2-CH3), 1.85 (m, 2H, --CH2ME), 1.10 (t, 3H, -CH3), 7.55- 7.83 (m, 3H, Ar-H); 13C NMR (100 MHZ) δ 163.1 (C- 1), 166.9 (C- 2), 129.2 (C-3), 131.8 (C-4), 121.5 (C-5), 136.1 (C-6), 124.0 (C-7), 146.2 (C-8), 23.1 (C-9), 13.4 (C-10), 13.4 (C-11), 175.1 (C-12C=O acetyl), 20.3 (C-13 CH3CO), MS m/z 275 (M+, 10), 276 (M+1, 33); Anal. Calcd. For C13H13N3O4 (275): C, 56.72; H, 4.76; N, 15.27; found: C, 53.85; H, 4.90; N, 15.40.
Ethyl 2-(6-nitro-2-propylquinazolin-4-yloxy) acetate (6)
To a mixture of 4 (2.33 g, 10 mmol) and ethyl chloroacetate (1.22 g, 10 mol,) in dry acetone (20 ml), anhydrous potassium carbonate (1.4 g, 10 mol,) was added and refluxed on a water-bath for 10 h. The reaction mixture was washed with water (3x100 ml). The organic material was extracted with ether (2x100 ml) and left to be evaporated slowly. The solid obtained was crystallized from ethanol to furnish 6. (2.23g, 70%); pale-yellow crystals; m.p: 165-167 oC; IR (KBr), ν (cm-1): 2900, 2920, 2880 (C-Haliphatic),1659 (C=Oester), 1615(C=N); 1H NMR (400 MHZ, DMSO-d6) δ: 1.25-1.34 (m, 6H, 2CH3-CH2-), 1.90(2H, -CH2-CH2-CH 3), 1.75 (m, 2H, - -CH2ME), 4.27 (q, 2H, -OCH2-CH3-), 4.78 (s, 2H, O-CH2-C=O), 8.19, 8.38 (m, 3H, Ar-H); 13C NMR (100 MHZ) δ 164 (C-1), 160 (C-2), 120.9 (C-3), 128.8 (C-4), 127.4 (C-5), 133.5 (C-6), 122.4(C-7), 147.1(C-8), 25.6(C-9), 14.6(C-10), 13.9 (C-11), 65.5(C-12OCH2), 170 (C-13C=O ester), 28.2(C-14), 15.5 (C-15), MS m/z 319 (M+, 22), 320 (M+1, 38); Anal. Calcd. For C15H17N3O5 (319): C, 56.42; H, 5.37; N, 13.16; found: C, 56.80; H, 5.80; N, 1350.80.
3-Hydroxy-6-nitro-2-propylquinazolin-4(3H)-one (7)
An equimolar mixture of benzoxazinone 3 (2.34 g; 10 mmol) and hydroxylamine hydrochloride (6.9 g; 10 mmol) in 20 mL of dry pyridine was heated under reflux for 4 h, left to cool and then poured into cold water with constant stirring. The solid product that separated out was filtered off, thoroughly washed with water, dried and then recrystallized from benzene to give compound 7.(1.86, 75%); white crystals; m.p: 230-231 oC; IR (KBr), ν (cm -1): 3530 (OH), 1680 (C=O), 1615(C=N); 1H NMR (400 MHZ, DMSO -d6) δ: 2.20(2H, -CH2-CH 2-CH3), 1.80 (m, 2H, -CH 2Me), 1.15 (t, 3H, -CH3), 11.20 (bs, 1H exchangeable with D2O, O-H), 7.80, 8.10 (m, 3H, Ar-H, quinazolone); 13C NMR (100 MHZ) δ 164.2 (C-1), 162.3 (C-2), 131.1 (C-3), 130.7 (C-4), 123.5 (C-5), 139.4 (C-6), 118.7 (C-7), 149.8 (C-8), 23.0 (C-9), 13.4 (C-10), 11.6 (C-11); MS m/z 249 (M+, 100), 250 (M+1, 12); Anal. Calcd. For C11H11N3O4 (249): C, 53.01; H, 4.45; N, 16.86; found: C, 53.30; H, 4.80; N, 16.90.
3-(Acetyloxy)-6-nitro-2-propylquinazolin-4(3H)-one (8)
1 mol of compound 7 (2.49g, 10 mmol) was heated under reflux in 30 mL of freshly distilled acetic anhydride for 3 h. The reaction solution was left to cool and the solid that deposited was filtered off, washed several times with light petroleum, dried and recrystallized from ethanol to afford the desired product 8. (1.9g, 65%); white crystals; m.p: 175-177 oC; IR (KBr), ν (cm-1): 1669 (C=OQuinazolinone), 1725 (C=Oester), 1614(C=N); 1H NMR (400 MHZ, DMSO-d6) δ: 2.02(2H, -CH2-CH2-CH3), 1.68 (m, 2H, --CH2ME), 1.28 (t, 3H, -CH3), 7.9 (s, 3H, COCH3), 7.80 -8.10 (m, 3H, Ar- H); 13C NMR (100 MHZ) δ 155.2 (C-1), 161 (C-2), 120 (C-3), 128 (C-4), 127.4 (C-5), 133.5 (C-6), 122 (C-7), 147 (C-8), 25.1 (C-9), 14.6 (C-10), 13.9 (C-11), 169.3 (C-12), 18.5 (C-13),; MS m/z 291(M+, 100), 292 (M+1, 12); Anal. Calcd. For C13H13N3O5 (291): C, 53.61; H, 4.50; N, 14.43; Found: C, 53.70; H, 4.80; N, 14.90.
General procedure for the synthesis of compounds 9a-c
A magnetically stirred solution of benzoxazinone 3 (2.34 g, 10 mmol) in glacial acetic acid (20 mL) maintained at 100 °C was treated with each of O-phenylendiamine (1.08 g, 10 mmol), O-aminophenole (1.09 g, 10 mmol) O -aminothiophenol (1.46 mL, 10 mmol). The resulting solutions stirred at 100 °C for additional 4 h before being cooled, poured into water (30 mL), and extracted with ethyl acetate (2 × 30 mL). The combined organic phases were washed with brine (2 × 30 mL) before being dried (Na2SO4), filtered, and concentrated under reduced pressure. The solid obtained were crystallized from AcOH to give compounds 9a-c.
3-(2-Aminophenyl)-6-nitro-2-propylquinazolin-4(3H)-one (9a)
(1.94 g, 60%); pale- yellow crystals; m.p: 164-165 oC; IR (KBr), ν (cm-1): 3360,3280 (NH2), 3051 (C- Haromatic),2920 (C-Haliphatic), 1675 (C=OQuinazolinone), 1620(C=N); 1H NMR (400 MHZ, DMSO-d6) δ: 2.00 (2H, - CH2-CH2-CH3), 1.70 (m, 2H, --CH2ME), 1.02 (t, 3H, -CH3), 5.2(s, 2H, D2O-exchangeable, NH2), 7.21-7.34 (m, 7H, Ar-H); 13C NMR (100 MHz) δ 164.0 (C-1), 164.8 (C-3), 133.0 (C-3), 131.0 (C-4), 124.1 (C-5), 135.1 (C-6), 117.5 (C-7), 148.5 (C-8), 22.1 (C-9), 13.9 (C-10), 11.8 (C-11),, 127.5 (C-12), 136.8 (C-13), 116.9 (C- 14), 125.9 (C-15), 119.0 (C-16), 120.0 (C-17); MS m/z 324 (M+, 100), 325 (M++1, 12); Anal. Calcd. For C17H16N4O3 (324): C, 62.95; H, 4.97; N, 17.27 %. Found: C, 63.20; H, 5.10; N, 17.40%.
3-(2-Hydroxyphenyl)-6-nitro-2-propylquinazolin-4(3H)-one (9b)
(2.5 g, 76 %); orange crystals; m.p: 170-172 oC; IR (KBr), ν (cm-1): 3360 (OH), 3040 (C-Haromatic),2929 (C-Haliphatic), 1680 (C=OQuinazolinone), 1625(C=N); 1H NMR (400 MHZ, DMSO-d6) δ: 2.20 (2H, -CH2- CH2-CH3), 1.85 (m, 2H, --CH2ME), 1.12 (t, 3H, - CH3), 7.22-7.85 (m, 7H, Ar-H), 11.81 (s, 1H, OH exchangeable with D2O); 13C NMR (100 MHz) δ 163.0 (C-1), 164.7 (C-3), 132.4 (C-3), 130.4 (C-4), 123.3 (C-5), 137.1 (C-6), 118.1 (C-7), 149.5 (C-8), 23.1 (C-9), 13.4 (C-10), 13.4 (C-11),, 127.3 (C-12), 138.4 (C-13), 115.1 (C- 14), 124.6 (C-15), 118.5 (C- 16), 121.1 (C-17); MS m/z 325 (M+, 60), 326 (M+ + 1, 22); Anal. Calcd. For C17H15N3O4 (325): C, 62.76; H, 4.65; N, 12.92 %. Found: C, 62.90; H, 4.80; N, 13.01%.
3-(2-Mercaptophenyl)-6-nitro-2-propylquinazolin-4(3H)-one (9c)
(1.7 g, 50 %); orange crystals; m.p: 210 -211 oC; IR (KBr), ν (cm-1): 3400 (SH), 3030 (C-Haromatic),2910 (C-Haliphatic), 1670 (C=OQuinazolinone), 1618(C=N); 1H NMR (400 MHZ, DMSO- d6) δ: 2.10 (2H, -CH2- CH2- CH3), 1.75 (m, 2H, --CH2ME), 1.10 (t, 3H, - CH3), 7.52-7.95 (m, 7H, Ar-H), 12.51 (s, 1H, SH exchangeable with D 2O,; 13C NMR (100 MHz) δ 163.2 (C-1), 164.1 (C-3), 132.0 (C-3), 130.0 (C-4), 123.1 (C-5), 136.1 (C-6), 118.5 (C-7), 149.0 (C-8), 22.9 (C-9), 13.2 (C-10), 11.4 (C-11),, 127.1 (C-12), 136.4 (C-13), 116.1 (C- 14), 125.6 (C-15), 119.5 (C- 16), 120.1 (C-17); MS m/z 341 (M+, 18), 342 (M+ + 1, 20); Anal. Calcd. For C17H15N3O3S (341): C, 59.81; H, 4.43; N, 12.31 %. Found: C, 60.00; H, 4.61; N, 12.44%.
General procedure for the synthesis of compounds 10a,b
A mixture of benzoxazinone 3 (2.342 g, 10 mmol) and the appropriate urea derivatives; urea (0.60g, 10 mmol) and/or thiourea (0.76 g, 10 mmol), in DMF (20 ml) was refluxed for 4 h. After the completion of reaction, the reaction mixture was poured into ice water to give a precipitate that was filtered off and recrystallized from ethanol to give 10a,b.
6-Nitro-4-oxo-2-propylquinazoline-3(4H)-carboxamide 10a
(1.66 g, 60%); pale-yellow crystals; m.p: 180-182 oC; IR (KBr), ν (cm-1): 3300 -3240 (NH2), 3050 (C- Haromatic),2950 (C-Haliphatic), 1675 (C=OQuinazolinone), 1640 (C=O), 1618(C=N); 1H NMR (400 MHZ, DMSO-d6) δ: 1.90 (2H, -CH2- CH2-CH3), 1.72 (m, 2H, -CH 2Me), 1.15 (t, 3H, -CH3), 4.5 (s, 2H, D2O-exchangeable, NH2), 7.50-7.70 (m, 3H, Ar-H); 13C NMR (100 MHz) δ 164 (C-1), 160 (C-2), 120.9 (C-3), 128.8 (C-4), 127.4 (C-5), 133.5 (C- 6), 122.4(C-7), 147.1(C-8), 25.6(C-9), 14.6(C-10), 13.9 (C-11), 168(C-12C=ONH2); MS m/z 276 (M+, 100), 277 (M+ + 1, 12); Anal. Calcd. For C12H12N4O4 (276): C, 52.17; H, 4.38; N, 20.28 %. Found: C, 52.20; H, 4.60; N, 20.65%.
6-Nitro-4-oxo-2-propylquinazoline-3(4H)-carbothioamide10b
(2g , 70 %); white crystals; m.p: 200-201 oC; IR (KBr), ν (cm-1): 3320, 3270 (NH2), 3000 (C- Haromatic ),2900 (C-Haliphatic), 1671 (C=OQuinazolinone), 1621(C=N), 1240 (C=S),; 1H NMR (400 MHZ, DMSO-d6) δ: 2.10 (2H, -CH2- CH2-CH3), 1.82 (m, 2H, -CH 2Me), 1.10 (t, 3H, -CH3), 5.4 (s, 2H, D2O-exchangeable, NH2), 7.90-8.50 (m, 7H, Ar-H); 13C NMR (100 MHz) δ 162 (C-1), 161 (C-2), 121.9 (C-3), 127.5 (C-4), 126.2 (C-5), 131. 5 (C-6), 120.4(C-7), 146.2(C-8), 24.6(C-9), 13.6(C-10), 11.9 (C-11), 172(C-12C=S); MS m/z 292 (M+, 32), 293 (M+ + 1, 18); Anal. Calcd. For C12H12N4O3S (292): C, 49.31; H, 4.14; N, 19.17%. Found: C, 49.70; H, 14.30; N, 19.40%.
General procedure for the synthesis of compounds 11a,b
An equimolar mixture of benzoxazinone 3 (2.34 g; 10 mmol)) and the proper hydrzanie; hydrazine hydrate (0.75 g, 10 mmol) and/or phenylhydrazine (1.08 g, 10 mmol), in ethanol was heated at refluxing temperature for 4 h. The solid that settled down on cooling was filtered off and crystallized from light petroleum 60/80 to give 11a, b.
3-Amino-6-nitro-2-propylquinazolin-4(3H)-one (11a)
(2.23g, 90%); pale- yellow crystals; m.p: 133-134 oC; IR (KBr), ν (cm -1): 3309, 3212 (NH2), 3010 (C- Haromatic), 2930 (C-Haliphatic), 1673 (C=OQuinazolinone), 1620(C=N); 1H NMR (400 MHZ, DMSO-d6) δ: 2.20 (2H, - CH 2-CH2-CH3), 1.80 (m, 2H, --CH2ME), 1.15 (t, 3H, -CH3), 5.50 (s, 2H, D2O -exchangeable, NH2), 7.20-7.80 (m, 3H, Ar-H); 13C NMR (100 MHz) δ 163.5 (C-1), 167.1 (C-2), 129.2 (C-3), 131.7 (C-4), 121.5 (C- 5), 136.7 (C-6), 124.3 (C-7), 146.4 (C-8), 23.0 (C-9), 13.4 (C-10), 12.6 (C-11); MS m/z 248(M +, 100), 249 (M+ + 1, 12); Anal. Calcd. For C11H12N4O3 (248): C, 53.22; H, 4.87; N, 22.57 %. Found: C, 53.55; H, 4.95; N, 22.90%.
6-Nitro-3-(phenylamino)-2-propylquinazolin-4(3H)-one (11b)
(2.75g, 85%); pale-yellow crystals; m.p: 192- 193 oC; IR (KBr), ν (cm-1): 3385 (NH), 3000 (C-Haromatic), 2910 (C-H aliphatic), 1675 (C=OQuinazolinone), 1622(C=N); 1H NMR (400 MHZ, DMSO-d6) δ: 2.10 (2H, -CH2-CH2-CH3), 1.70 (m, 2H, --CH2ME), 1.10 (t, 3H, -CH3), 8.90 (bs, 1H exchangeable with D2O, N-H), 7.50-8.80 (m, 8H, Ar-H); 13C NMR (100 MHz) δ 163.1 (C-1), 166.9 (C-2), 129.2 (C-3), 131.8 (C-4), 121.5 (C-5), 136.1 (C-6), 124.0 (C-7), 146.2 (C-8), 23.1 (C-9), 13.4 (C-10), 12.3 (C-11), 136.0 (C-12), 130.1 (C- 13), 128.4 (C-14), 132.1 (C- 15), 128.4 (C-16), 130.1 (C-17); MS m/z 324(M+, 100), 325 (M+ + 1, 12); Anal. Calcd. For C17H16N4O3 (324): C, 62.95; H, 4.97; N, 17.27; %. Found: C, 62.02; H, 5.10; N, 17.90%.
N-(6-nitro-4-oxo-2-propylquinazolin-3(4H)-yl)acetamide (12)
A solution of 11 (2.48 g, 10 mmol) and acetic anhydride (20 mmol) in glacial acetic acid (30 mL) was heated at refluxing temperature for 4 h. When the reaction mixture was left to cool and diluted with cold water, the solid which separated out was filtered off and recrystallized from ethanol to afford 12. (2 g, 70 %); yellow crystals; m.p: 170-172 oC; IR (KBr), ν (cm-1): 3487(OH), 3280 (NH), 3080 (C-Harom),2910 (C-Haliph), 1673 (C=OQuinazolinone), 1635 (C=Oamidic) 1610(C=N); 1H NMR (400 MHZ, DMSO-d6) δ: 2.30 (2H, -CH2-CH2-CH3), 2.00 (2, 3H, CH3-C=O), 1.70 (m, 2H, --CH2ME), 1.30 (t, 3H, -CH3), 8.00-7.85 (m, 3H, Ar-H), 6.50 (bs, 1H exchangeable with D2O, NH), 8.90 (bs, 1H exchangeable with D2O, OH); 13C NMR (100 MHz) δ 155.2 (C-1), 161 (C-2), 120 (C-3), 128 (C-4), 127.4 (C-5), 133.5 (C-6), 122 (C-7), 147 (C-8), 25.1 (C-9), 14.6 (C-10), 13.9 (C-11), 173.0(C-12 NHCO), 12.0(C-13COCH3); MS m/z 290(M+, 100), 291 (M+ + 1, 12); Anal. Calcd. For C13H14N4O4 (290): C, 53.79; H, 4.86; N, 19.30 %. Found: C, 53.90; H, 4.95; N, 19.90%.
Methyl (6-nitro-4-oxo-2-propylquinazolin-3(4H)-yl)carbamodithioate (13)
An aqueous solution of sodium hydroxide (12 mL, 24 mmol, 2 N) was drop- wise added to a stirred mixture of the amine 11 (2.48 g, 10 mmol) and carbon disulfide (1.5 mL, 24 mmol), in DMSO (20 mL), in an ice bath, over a period of 30 min. Then the reaction mixture was stirred for additional 3 h, afterwards, dimethyl sulphate (1.9 mL, 20 mmol) was gradually added with continuous stirring in for 2 h. Then the reaction mixture was diluted with ice- cold water (40 mL) to give solid deposits which were filtered, washed with water, dried and crystallized from acetonitrile to yield compound 13. (2 g, 60%); yellow crystals; m.p: 145-146 oC; IR (KBr), v (cm-1): 3280, 3150 (N-H), 3060 (C- Haromatic), 2980, 2900, 2870 (C-Haliphatic ), 1670 (C=O), 1610 (C=N, N- H), 1450 (C=S).; 1H NMR (400 MHZ, DMSO-d6) δ: 2.50 (s, 3H, SCH3), 2.10 (2H, -CH2-CH2-CH3), 1.65 (m, 2H, --CH2ME), 1.15 (t, 3H, -CH3), 7.20-7.80 (m, 3H, Ar-H), 9.95 (bs, 1H exchangeable with D2O, N-H); 13C NMR (100 MHz) δ 155.2 (C-1), 161 (C-2), 120 (C-3), 128 (C-4), 127.4 (C-5), 133.5 (C-6), 122 (C-7), 147 (C-8), 25.1 (C-9), 14.6 (C-10), 13.9 (C-11), 179.3 (C-12CS), 12.2 (C-13 SCH3); MS m/z 338(M+, 100), 339 (M+ + 1, 12); Anal. Calcd. For C13H14N4O3S2 (338): C, 46.14; H, 4.17; N, 16.56 %. Found: C, 46.30; H, 4.25; N, 16.90%.
3-(Benzylideneamino)-6-nitro-2-propylquinazolin-4(3H)-one (14)
A mixture of 11 (2.48 g, 10mol) and benzaldehyde (0.01 mol) in 60 ml of ethanol, containing a few drops of piperidine as a catalyst, was refluxed for 4h. The solid separated out upon cooling was filtered of and recrystallized from benzene to produce compound 14. (2.7g, 80%); white crystals; m.p: 180-181 oC; IR (KBr), v (cm-1): 3020 (C- Haromatic ),2900(C-Haliphatic), 161676 (C=O), 1610 (C=N); 1H NMR (400 MHZ, DMSO-d6) δ: 2.20 (2H, -CH2-CH2-CH3), 1.85 (m, 2H, --CH2ME), 1.10 (t, 3H, -CH3), 7.40-8.20 (m, 8H, Ar-H), 8.80 (s, 1H, N=CH); 13C NMR (100 MHz) δ 163.1 (C-1), 166.9 (C-2), 129.2 (C-3), 131.8 (C-4), 121.5 (C-5), 136.1 (C-6), 124.0 (C-7), 146.2 (C-8), 23.1 (C-9), 13.4 (C-10), 13.4 (C-11),164.5( C-12C=N), 136.0 (C-13), 130.1 (C- 14), 128.4 (C-15), 132.1 (C-16), 128.4 (C-17), 130.1 (C-18); MS m/z 336(M+, 100), 337 (M+ +1, 18); Anal. Calcd. for C18H16N4O3 (336): C, 64.28; H, 4.79; N, 16.66 % . found: C, 64.30; H, 4.90; N, 16.90%.
Results and discussion
As part of our concern in the organic synthesis of quinazolinone derivatives [18]. We produced a new chemosensor carrying quinazolinone moiety. As displayed in (Scheme 1), 2-amino-5-nitrobenzoic acid (1) was reacted with butyryl chloride, in dry pyridine, to yield 2 -(butyramido) -5- nitrobenzoic acid (2). Micro analytical and spectral data were used for structure elucidation of the anilide 2. IR spectrum showed absorption bands at v 1730 and 1640 cm-1 which are characteristic for both C=O carboxylic acid and amide functions stretching vibration, respectively. 1H NMR spectrum of the anilide 2 exposed two deuterium-exchangeable, protons at δ 8.95 and 11.80 related to N-H and CO2H. Mass spectrum showed the molecular ion peak at m/z 252 which is coincident with the prospective anilide formula.
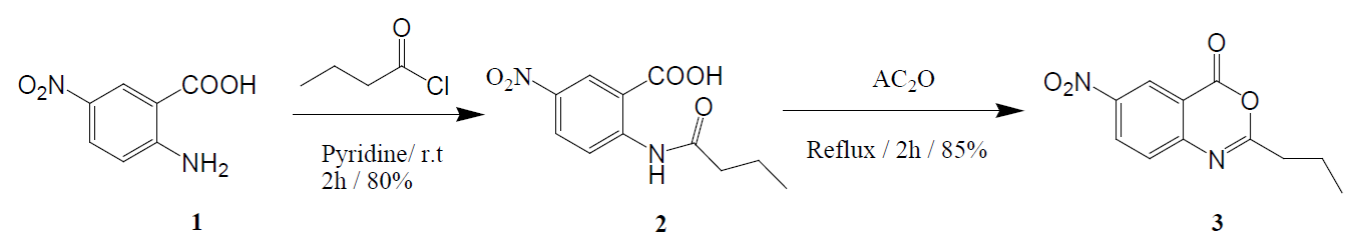
Scheme 1
Synthesis of 6-nitro-2-propyl-4H-benzo[d][1,3]oxazin-4-one (3).
Using acetic anhydride, intramolecular cyclization for of the anilide 2 was performed leading to our target 6-nitro-2-propyl-4H-benzo[d][1,3]oxazin-4-one (3) as a dynamic benzoxazinone moiety with electronically unsaturated character [19]. IR spectra of compound 3 was very good indication for absence of both carboxylic and amide functions. On the other hand, a characteristic absorption band was observed at ν 1750 cm-1 signifying C=O function of 3,1-benzoxazin-4-ones. Mass spectrum gave a molecular ion peak at m/z 234 which is matching with the predictable molecular formula acquired through loosing of one molecule of water from the anilide 2. These results are deemed confirmatory for the postulation that cyclization process implicated both carboxylic and amidic functions (Scheme 1).
Reaction of 6-nitro-2-propyl-4H-benzo[d][1,3]oxazin-4-one (3) with some selected nitrogen nucleophiles, namely, formamide, hydrazine hydrate, hydroxylamine hydrochloride, O-phenylendiamine, O-aminophenol, O-aminothiophenol, urea and/or thiourea furnished a variety of 4(3H)-quinazolinones via aminolysis at position 3 (Scheme 2). It is expectant that the reactions managed through nucleophilic ring opening ring closure (RORC) process accompanied by ring oxygen replacing with nitrogen. Oxazinone ring splitting takes places by the attack of nitrogen nucleophile followed by cyclization on the highly electrophilic SP2 hybridized carbonyl carbon of the intermediate to give our coveted quinazolinones [20].
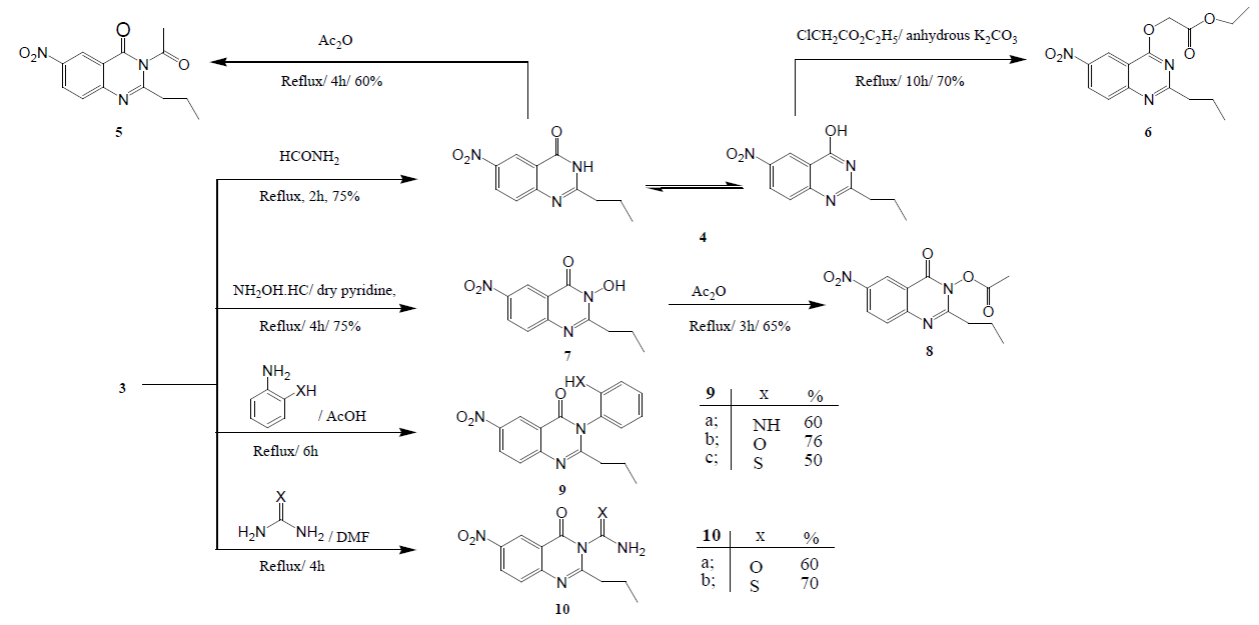
Scheme 2
Synthesis of quinazolinones 3 - 10.
Fusion of the benzoxazinone 3 with extra amount of formamide afforded 6-nitro- 2-propylquinazolin-4(3H)-one (4). Structure of compound 4 was elucidated from its correct elemental analysis together with its IR spectrum which showed strong absorption bands at ν 3280, 3150, 1670, and 1622 cm-1 related to NH (bonded and non-bonded), C=O, and C=N, respectively. Quinazolinone 4 showed, in solution, lactam-lactim dynamic equilibrium. In polar solvents, the lactam form is more predominant than the lactim one, hence with decreasing the solvent polarity, the lactim form becomes more predominant [21].
The existence of compound 4 in the lactam-lactim tautomeric equilibrium was demonstrated via its reaction with acetic anhydride where it undergo N-acetylation (through the lactam form) to yield compound 3-acetyl-6-nitro-2-propylquinazolin-4(3H)-one (5), but, it upon alkylation with ethyl chloroacetate the ester ethyl 2-(6-nitro-2-propylquinazolin-4-yloxy)acetate (6) was produced (through the lactim form) (Scheme 2). The 1H-NMR spectra of compounds 5 and 6 afforded signals at δ 2.10 ppm for three protons related to compound 5 acetyl group (CH3-C=O) in addition to δ 1.20- 1.30 and 4.00-4.10 which proved the existence of the ester group protons in compound 6 (3H, CH3-CH2-), (2H, -OCH2-CH3-.
Aiming to expand the synthetic efficiency of our newly synthesized benzoxazinone 3, reaction with hydroxylamine hydrochloride was studied as convenient route to the synthesis of 3-hydroxy-6-nitro-2-propylquinazolin- 4(3H) -one (7). Structure of compound 7 was proved from its IR spectrum which showed strong absorption bands at ν 3530 and 1680 cm−1 due to both (OH and C=O) groups, respectively. The 1H NMR spectrum showed the D 2O exchangeable proton for the OH group at 11.50 ppm. Reactions of the ultimate compound 7 with acetic anhydride yielded 3-(acetyloxy) -6-nitro-2-propylquinazolin -4(3H)-one (8) which is a convenient intermediate for various organic synthesis. Structure of compound 8 was proved from its IR spectrum which showed a notable absorption bands at ν 1614, 1670 and 1725 due to C=N, C=O (amide) and C=O (ester) groups, respectively with the lake of ν OH band. Mass spectrum of both compounds 7 and 8 exhibited their proposed molecular ion peaks at m/z 249, 291 respectively as base beaks.
The aforementioned findings prompted us to carry out the reaction of 3 with some additional N-nucleophiles. So, reaction of compound 3 with an equimolar quantities of 1,4-N,N, 1,4-N,O, and 1,4-N ,S-nucleophiles, namely, O-phenylendiamine, O- aminophenol and O-aminothiophenol, furnished the corresponding 3-substituted aminoquinazolinones 9a-c. 1H NMR spectra of products 9a-c exhibited the integral count of seven aromatic protons at their convenient chemical shift area.13CNMR spectra of both compounds 9a-confirmed the proposed structures exhibiting the expected count of carbon atoms. The mass spectrometry was an outstanding tool to demonstrate the suggested structures for compounds 9a-c showing their molecular ion peaks in convenience with their proposed molecular formulas.
In the same sense, equimolar remediation of compound 3 with 1, 3-binnucleophiles such as urea and thiourea in boiling DMF furnished smoothly the anticipated quinazolinone derivatives 10a,b. Analytical and spectral data of these products were found in similarity with the proposed formula. IR spectra of both 10a,b compounds showed the C=O stretching vibrations at 1671, 1675 cm-1 and the C=S stretching vibrations at 1220-1050 cm-1 in addition to NH2 stretching vibrations at 3330, 3270 cm-1. The 1H NMR spectra of compounds 10a, b demonstrated the appearance of the emergence of new singlets signals assigned to the characteristic NH2 groups protons at 6.2 and 6.5 ppm for both 10a and 10b, respectively. 13H NMR spectral data displayed a characteristic signal at δ 177.1 and 178.2 ppm attributed to both C=O and C=S carbons, respectively. In addition, mass spectrum represents a good guide to 10a, b structure and showed the molecular ion peak at m/z 276 and 292 which corresponds to the suggested molecular formulas.
Benzoxazinone 3 was reacted with hydrazine hydrate and /or phenyl hydrazine in boiling ethanol, 3-amino-6-nitro-2-propylquinazolin-4(3H)-one (11a) and 6-nitro-3-(phenylamino)-2-propylquinazolin-4(3H)-one (11b) were produced in a respective yield. 3-Aminoquinazolinone 11a used as many-sided constructing bulk in synthetic heterocyclic chemistry. IR spectrum of the amine 11 exhibited a specific stretching bands at ν 3309 and 3212 cm -1 related to symmetric and asymmetric vibrations of NH2 group. The absorption bands due to C=O group, for ideal quinazolin-4-ones, was observed at ν 1673cm -1. These outcomes are in accordance with 1H NMR spectral data of compounds 11 which showed a broad singlets chemical shifts at δ 5.50 due to deuterium-exchangeable protons of NH2. In addition, the mass spectra of compound 11a revealed its molecular ion peak at m/z 248 as base peak. All spectral data of 6-nitro-3-(phenylamino)-2-propylquinazolin-4(3H)-one (11b) were fully consistent with its suggested formula (Scheme 3).
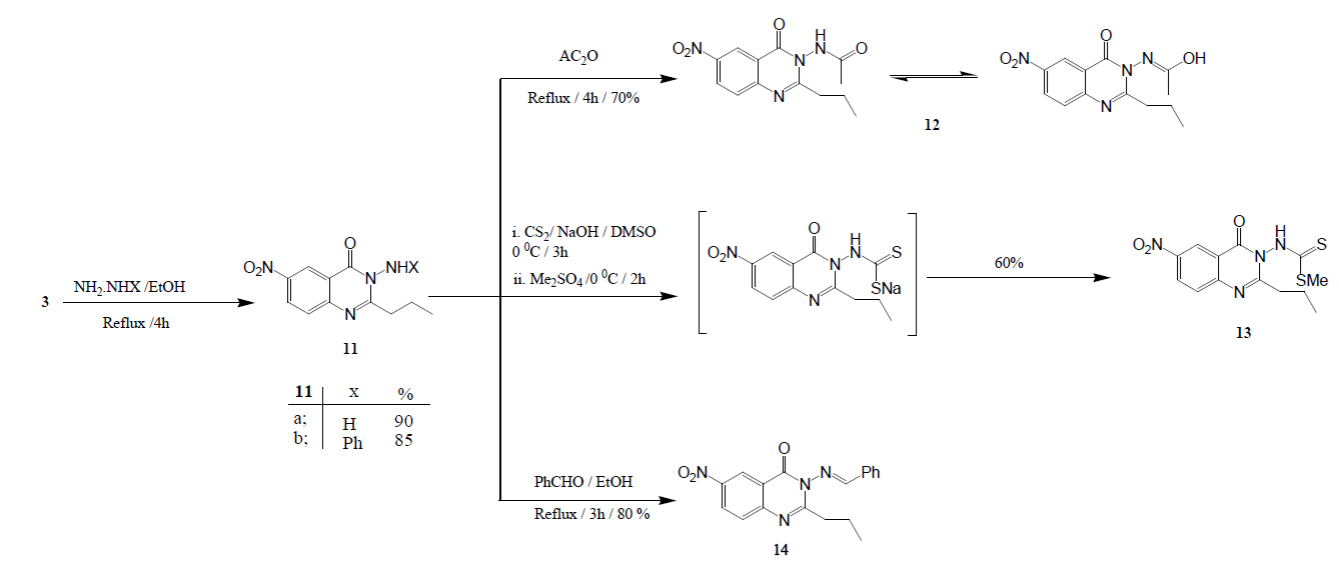
Scheme 3
Synthesis of quinazolinones 11-14.
N-(6- nitro-4-oxo-2-propylquinazolin-3(4H)-yl)acetamide (12) was obtained on refluxing of quinazolinone 11a with acetic anhydride. The IR spectrum of compound 12 revealed strong absorption bands assigned to C=N, two C=O group, NH, and OH, respectively. 1H NMR spectrum of compound 12 revealed that it exists in solution in a keto-enol tautomerism of an amide functionality, as it showed two singlet signals at δ 6.50 and 8.90 attributable to NH and OH of the keto and the enol forms, respectively.
The carbothioamides 13 was targeted because of its expected biological activity [22]. Consequently, reacting of the amine 11a with carbon disulfide, in existence of sodium hydroxide as base catalyst, and in situ methylation of the non-separable sodium dithiocarbamide salt, with the use of dimethyl sulfate, yielded Methyl(6-nitro-4-oxo- 2-propylquinazolin-3(4H)-yl)carbamodithioate (13) . Structure of compound 13 was elucidated from its IR spectrum which showed a strong absorption bands at 1450 and 1670 related to υ max C=S and C=O respectively and freed from the NH 2 band. 1H NMR spectrum of the product showed a characteristic singlet chemical shift at δ 2.54 due three protons of SCH3. Mass spectrum exhibited a molecular ion peak at m/z 338, confirming the proposed molecular formula. The amine 11 a was condensed with benzaldehyde to give the corresponding Schiff’s bases 14 (Scheme 3) which could be utilized as multilateral building bulk in a variety of heterocyclic synthesis. It will be of interest in our future project to discuss the behavior of azomethine 14 which includes an activated azomethine group (-N=CH) [23]. Spectral data of the product pointed to the disappearance of NH2 function, indicating its embodiment in the condensation reaction. IR spectra of compounds 14 showed a strong absorption bands at 1610, 1676; related to ν C=N and ν C=O, respectively in addition of lacking any absorption band due to NH 2. 1H NMR spectrum of the trio gave specific chemical shift signals of azomethine proton (N=C-H) observed at δ 8.80. Mass spectrum exhibited a molecular ion peak at m/z 336 as base peak, confirming the proposed structure.
Colorimetric study
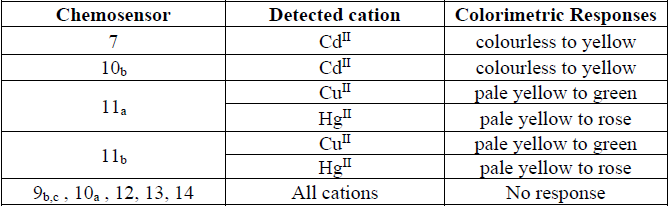
The colorimetric study was applied for 2.5x10-5 M of all the synthesized compounds, which were prepared in DMF- water (1:10). As is clear from the (Table 1), Compounds 9b, c, 10a, 12, 13 and 14 did not give any changes of color after addition of any metal cation. The chemosensor 10b and 7 showed the color change from colorless to yellow when adding Cd II ion and the intensity of color was increased with further addition. The chemosensors 11a,b exhibited notable color change from pale yellow to green when adding Cu II ion which gets more colour intensity on further addition of CuII cation. The same chemosensor 11a,b showed a remarkable colour shift to rose with addition of HgII ion and with further addition, the colour intensity increased. This important color changes can be easily applied for detection CuII, CdII, and HgII ions in an aqueous mediums and biological samples.
Colorimetric responses of selected chemosensors toward various cations.
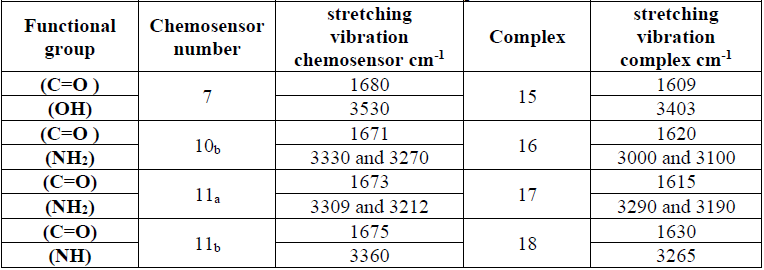
FT-IR spectrum of the synthesized compounds towards their complexes
The FT- IR comparison of some of our newly synthesized chemosensor 7, 10b and 11a, b versus their related complexs 15, 16, 17 and 18 were identified as shown in the (Table 2). The FT- IR spectrum changes of our produced complexs confirm that our primary amine, carbonyl groups, thiocarbonyl and hydroxyl group are sharing in the binding with our targeted metal cations.
FT-IR variation between chemosensors and formed complexes.
UV-vis absorption spectroscopy study
The absorption response of complexes 15, 16, 17, and 18 were examined in the presence of different solvents, for instance, ethanol, dimethylformamide, and acetonitrile. The chemosensor 7, 10b, 11a,b displayed good absorption intensity in the dimethylformamide solution contrast to other solvents and so dimethylformamide was used as a solvent for all UV-Vis spectral studies because of its good absorbance shift and absorption intensity [24]. Metal ions were prepared as aqueous solutions of 0.02 M nitrate salts in distilled water. Chemosensor stock solutions of 7, 10b, 11a,b (0.02 M) were dissolved in dimethylformamide. By using a micropipette, we prepared and diluted various concentrations of metal ions to 2.5 x 10-5 M with the same solution. The diluted chemosensors were added to various concentrations of metal ions. Complexes 17-CuII, 17-HgII, 18-CuII, 18-HgII, 15-CdII and 16-CdII showed the main absorption peaks (λmax) at 560 nm, 670 nm, 505 nm, 695 nm, 585 nm and 562 nm, respectively. The UV titration study was established as UV absorbance of chemosensors as function of cations gradual addition (Fig. 1). Also, calibration curves of absorbance intensity of different concentrations of ions were established (Fig. 2).
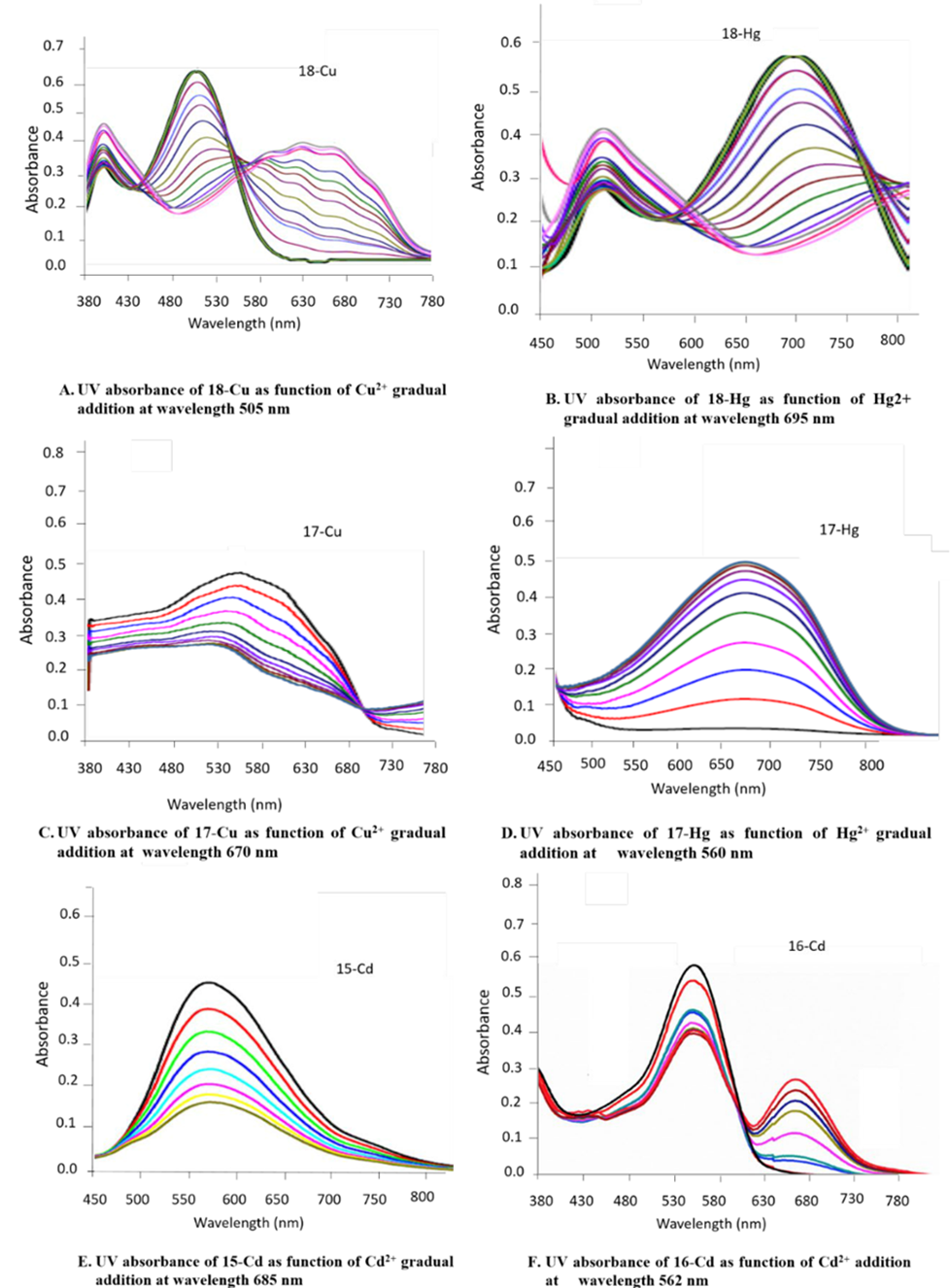
Fig 1
UV absorbance of chemosensors as function of cations gradual addition at λ max wavelength 560 nm.
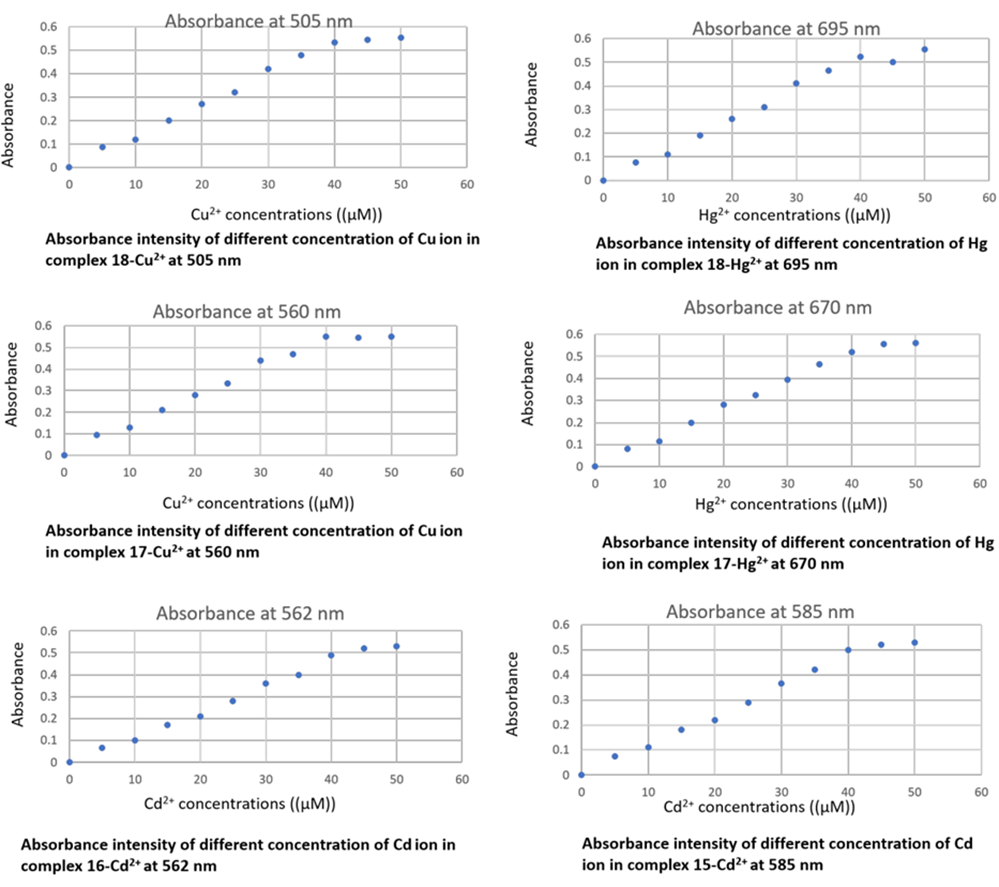
Fig 2
Calibration curves of Absorbance intensity of different concentrations of ions.
Determination of association constant by Benesi-Hildebrand equation
Changes in absorbance intensity upon addition of various competitive cations along with chemosensor ions showed no interference on the sensing ability of chemosensors 7, 10b, 11a,b for Cu II, CdII, and HgII cations (Fig. 3). The binding constant (Ka) was evaluated for the chemosensor 7, 10b, 11a,b from the equation of Benesi-Hildebrand [25] on applying UV-Vis absorption data (Fig.1,2) and it was calculated to be 6.5x104 M-1 for complex 15-Cd , 3.6 x 103 M-1 for complex 17-Cu, 5.2 x 103 M-1 for complex 17-Hg, 6.1 x 103 M-1 for complex18-Cu and 7.4 x 102 M-1 for complex181-Hg . The K 1was calculated using formula.
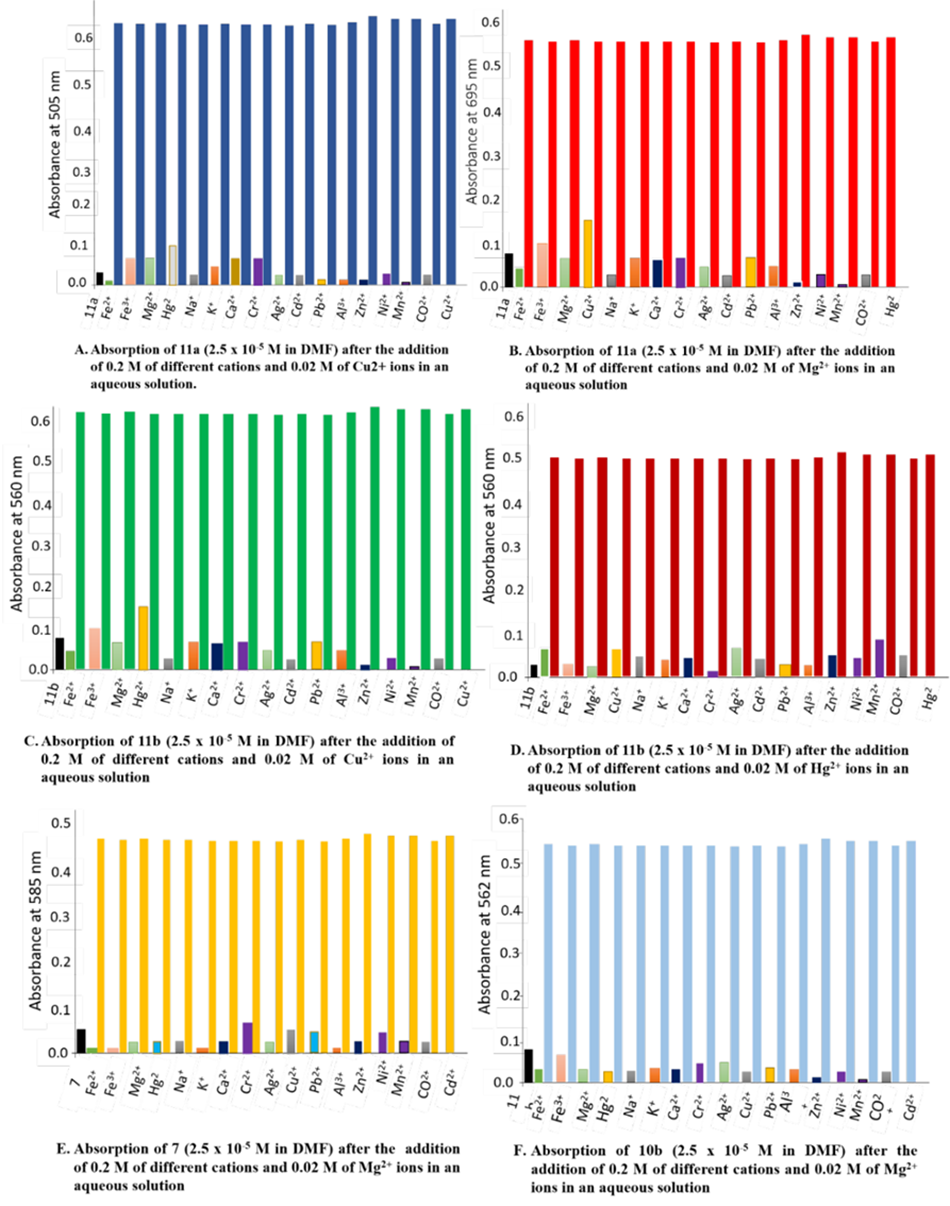
Fig 3
Absorbance of chemosensors 7, 10b, 11a and 11b after the addition of 0.2 M of different cations and 0.02 M of cation substrate in an aqueous solution.
Where, Ao is absorption considered in the absence of metal, A is the absorption at an intermediate, Amax is the absorption at a saturation concentration, K is complexation constant, and [M] is concentration of investigated metal ions and n is the stoichiometric ratio. The stoichiometry calculation data of the complexes 15, 16, 17, and 18 were established to be 1:1 stoichiometry for the investigated metals.
Selectivity and specificity
Selectivity and specificity were examined by one measurement of absorbance chemosensors with investigated cations (CuII, CdII and HgII) and another measurement of absorbance chemosensors with investigated cations (CuII, Cd II and HgII) in the presence of competitive cations FeIII, FeII, MgII, NaI, KI, AgII, CrII, AlIII , PbII, CaII, ZnII, NiII, MnII and CoII (Fig. 3).
The suggested mechanism
According to UV-Vis absorption analysis and the FT-IR spectrum of compounds 7, 10b, and 11a,b towards their complexes 15, 16, 17 and 18 respectively, the binding positions were labeled in chemosensor 7, 10b, and 11a,b. Some predictable observations can be inferred. The variations in the FT-IR frequencies of 17 and 18 suggested that amino and carbonyl groups are participating in the binding with metal ions as shown in Table 3 (Fig. 4, 5, 7, and 8) . In the aqueous medium, the nucleophile chemosensors 11a,b attacked cations by lone pairs of electrons from amino and carbonyl groups to form five-membered ring which have high stability and a low amount of ring strained [26]. The change of the FT-IR frequencies of complex 16 indicated that CdII coordinated with oxygen atom of carbonyl group and C=S group as showen in Table 3 (Fig. 6). The change of the FT-IR frequencies of complex 15 indicated that the nucleophile chemosensor 7 attacked cations through OH and C=O groups lone pairs of electrons to form a five-member ring, Table 3 (Fig. 9).
Proposed binding mechanism of different chemosensors.
Conclusion
The current study established new chemosensors quinazolinones 7, 10b and 11a,b for the quantitative determination of CuII, CdII at exact wavelengths in an aqueous medium, and in blood samples. Similarly, chemosensors 11a,b have been synthesized for determination of HgII ion in an aqueous medium and the environmental samples like water samples.
References
1. Anu Kundu, P.S.; Hariharan, K. P.; Savarimuthu, P. A. Sensors and Actuators B. 2015, 206, 524-530.
2. Kevin, J. B.; Colin, L. M.; Ashley, I. B. Nat. Rev. Drug Discov. 2004, 3, 205-2014.
3. Robert, R. L.; Alfred, M. B.; Harry, A. R.; Jean, P.B. CLIN. CHEM. 1994, 40, 1391-1394.
4. Ullah, I. K. ; Rahim, M. ; Haris, M. J. Chem. Soc. Pak. 2016, 38, 177-185.
5. Ivan, S. ; Irina, K. ; Trajce, S.; Juli, J. Microchem. J. 2008, 89,42-50.
6. Leermakers, M. W.; Baeyens, P.; Horvat, M. Trends Anal.Chem. 2005, 24, 383-392.
7. Weiying, L.; Lin, Y.; Wen, T.; Jianbo, F.; Lingliang, L. Chem. Eur. J. 2009, 15, 1030 -1035.
8. Takunori, K.; Seiji, N.; Masatoshi, M. Anal. Sci. 1990, 6, 623-626.
9. Paramjit, K.; and Divya, S. Dyes Pigm. 2011, 88, 296-300.
10. Ha, N.; Wa, Wen. ; X. R.; Jong, S. K.; Juyoung, Y. Chem. Soc. Rev. 2012, 41, 3210-3244.
11. Meng, Li.; Hai-Yan, Lu. ; Rui-Li, Liu. ; Jun-Dao, C.; Chuan-Feng, C. J. Org. Chem. 2012, 77, 3670-3673.
12. Umesh, F. ; Anu, S. Suban, K. S. ; Narinder, S. ; Ratnamala, B. ; Anil, K. RSC Adv. 2014, 4, 39639-39644.
13. Xue-Jiao, B.; Jing, R.; Jia, Z.; Zhi-Bin, S. Heterocycl. Commun. 2018, 24,135-139.
14. Chunliang, L. ; Zhaochao, X. ; Jingnan, C. ; Rong, Z. ; Xuhong, Q. J. Org. Chem . 2007, 72, 3554-3557.
15. Maher, A. E. ; Khalid, M. D. ; Sameh, A. R. ; Fakhry, A. E. Pharmaceuticals. 2011, 4, 1032-1051.
16. Ailin, Y.; Chunling, Z.; Zhengyu, Z.; Lu, Y.; Chao, L.; Haibo, W. Fluoresce. 2014, 24, 557-561.
17. Pravin, N. B.; Pranila, B. T.; Ganapati, S. S. Dyes Pigm . 2016, 134, 276-284.
18. Ayman, M. F. E.; Mohamed, M. H.; Mohamed, A. Der Pharma Chem. 2011, 3, 1-12.
19. Maher, A. E.; Mohamed, E. A.; Jehan, M. M. J. Heterocyclic Chem. 2016, 53, 95-101.
20. Ghorab, M.M.; Hassan, A.Y. Phosphorus, Sulfur and Silicon. 1998, 141, 251-261.
21. Joule, A. ; Mills, K. ; Smith, F. Heterocyclic Chemistry Springer, 3rd Ed., 1995.
22. Paul, G. ; Tinku, B. ; William, P. M. ; Alexander, J. M. ; George, C. P. ; James D. ; Shauna, B. ; Ashley, M. D. J. Med. Chem. 2006, 49, 684-692.
23. Ashok, K.; Chatrasal, S. R. E. J. Med. Chem. 2011, 44, 83-90.
24. Venkatadri, T.; Darshak, R. T. Anal. Chim. Acta. 2017, 972, 81-93.
25. Lu, C.; Xu, Z.; Cui, J.; Zhang, R.; Qian, X. J. Org. Chem . 2007, 72, 3554-3557.
26. Wiberg, K. B.; Lampman, G. M.; Ciula, R. P.; Connor, D. S.; Schertler, P.; Lavanish, J. Tetrahedron 1965, 21, 2749-2796.
Author notes
*Corresponding author: Mohamed M. Hassan, email: [email protected]

 cygnusmind
cygnusmind